Abstract
Human small airway epithelial cells (HSAEC) form the boundary between the external environmental allergens and the internal lung milieu. Mast cells are present in human lung tissue interspersed within the pulmonary epithelium and can secrete a host of pre- and newly formed mediators from their granules, which may propagate small airway inflammation. In this study, tryptase stimulation of HSAEC increased membrane-associated, calcium-independent phospholipase A2γ (iPLA2γ) activity, resulting in increased arachidonic acid and PGE2 release. These responses were inhibited by pretreating HSAEC with the iPLA2-selective inhibitor bromoenol lactone. The tryptase-stimulated PGE2 production was inhibited by treating HSAEC with the cyclooxygenase (COX)-1-selective inhibitor SC-560 and the nonselective COX inhibitor aspirin but not by the COX-2-selective inhibitor CAY10404, indicating that the early release of arachidonic acid is metabolized by constitutive COX-1 to form PGE2 in tryptase-stimulated HSAEC. Additionally, platelet-activating factor production and neutrophil adherence to tryptase-stimulated HSAEC was also increased. This complex response can set up a cascade of inflammatory mediator production in small airways. We speculate that selective inhibition of iPLA2γ-mediated phospholipid hydrolysis may prove beneficial in inflammatory airway diseases.
Keywords: prostaglandin E2, arachidonic acid, bromoenol lactone
the epithelium lining the respiratory tract in humans is composed of ciliated, nonciliated, and basal cells. These are the first cells to be exposed to an inhaled allergen (17). Damage to the epithelial cell lining results in cilial dysfunction, loss of cellular integrity, and penetration of allergens into the airways. Loss of epithelial integrity is a major feature of asthma pathogenesis and is thought to be an important contributor to the development of airway hyperresponsiveness (8). Resident mast cells in the pulmonary interstitium are important facilitators in the epithelial cell response to external injury. They secrete a host of mediators from the preformed granules including tryptase, chymase, and carboxypeptidase, which affect tissue remodeling and inflammatory cell recruitment (8, 25). In addition, mast cells also contain histamine, a vasoactive amine with potent effects on vascular permeability. Platshon and Kaliner (34) have concluded that histamine stimulation of human lung tissue leads to increased synthesis of PGF2α and cGMP in response to H-1 receptor stimulation and of cAMP through H-2 receptor stimulation. The rapid degranulation and release of tryptase following mast cell exposure to allergens provides a signal for activation of phospholipases, initiation of vascular change, and mobilization and recruitment of effector inflammatory cells (8, 17, 25, 34).
Previous studies from our laboratory (35, 36) have demonstrated that tryptase stimulation of endothelial cells can lead to activation of a calcium-independent phospholipase A2 (iPLA2), which hydrolyzes membrane phospholipids resulting in the stoichiometric production of a fatty acid, including arachidonic acid, and a lysophospholipid. In airway epithelial cells, arachidonic acid is further catabolized to eicosanoids, most typically PGE2. PGE2 acts on various E-prostanoid (EP) receptors and has been implicated in modulating human airway smooth muscle function. Activation of EP1 and EP3 receptors mediates airway smooth muscle contraction, whereas stimulation of EP2 receptors produces muscle relaxation (20).
The iPLA2 family of enzymes is made up of at least seven members (37). The first mammalian iPLA2 described was iPLA2β (group VIA PLA2; Ref. 14). In 2000, two independent laboratories identified a novel iPLA2, iPLA2γ (group VIB PLA2), which is constitutively membrane bound and possesses a COOH-terminal serine-lysine-leucine (SKL) peroxisomal targeting sequence (26, 28, 42). Multiple forms of iPLA2γ exist, and the expression of iPLA2γ is subject to rigorous control at many levels although the precise mechanisms for this are still not completely understood.
iPLA2γ protein expression has been confirmed in the mouse (28) and rat heart (27), rat liver (47), and human platelets (6) by immunoblot analysis. Overall, iPLA2γ expression appears to be widespread, but commercially available tools for the study of iPLA2γ (e.g., antibody, specific inhibitors, validated small interfering RNA, etc.) are only now becoming available. Both iPLA2β and iPLA2γ are sensitive to inhibition by bromoenol lactone (BEL) at low micromolar concentrations (26). However, other pharmacological PLA2 inhibitors, such as arachidonyl trifluoromethylketone (AACOCF3) and methyl arachidonyl fluorophosphonate (MAFP), which inhibit cytosolic iPLA2β at low micromolar concentrations (1, 21), do not inhibit microsomal iPLA2γ (10). Furthermore, Jenkins et al. (18) demonstrated that separation of racemic BEL into the R- and S-enantiomers provides a mechanism to discriminate between iPLA2γ and iPLA2β activity (i.e., iPLA2γ is ∼10-fold more sensitive to R-BEL than to S-BEL, and iPLA2β is 10-fold more sensitive to S-BEL than to R-BEL; Ref. 18).
This study examines the effect of tryptase stimulation on human small airway epithelial cell (HSAEC) PLA2 activity and the subsequent production of arachidonic acid, PGE2, and platelet activating-factor (PAF) and discusses the implications of these findings in small airway inflammation.
MATERIALS AND METHODS
HSAEC were obtained from Lonza Walkersville (Walkersville, MD). BEL, CAY10404, and SC-560 were obtained from Cayman Chemicals (Ann Arbor, MI). Recombinant human skin β-tryptase was obtained from Promega (Madison, WI). PX-18 was a gift from Richard Berney Associates (Bethesda, MD). CV3988 was purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were purchased from Sigma-Aldrich.
Culture of epithelial cells.
HSAEC were grown to confluence in small airway basal medium (SABM; Lonza Walkersville) and incubated at 37°C with an atmosphere of 95% O2-5% CO2. Cells were passaged using subculture pack (Lonza Walkersville) in a 1:3 ratio. Cells from passages 3–4 were used for experiments.
PLA2 activity.
Cells were grown to confluence in 100-mm culture dishes. At the end of each incubation period, media was removed and immediately replaced with ice-cold buffer containing, in mmol/l, 250 sucrose, 10 KCl, 10 imidazole, 5 EDTA, and 2 dithiothreitol, with 10% glycerol (pH 7.8). The cells were removed from the tissue culture plate by scraping, and the suspension was sonicated on ice for six bursts of 10 s. For separating the cells into membrane and cytosolic fractions, the sonicate was centrifuged at 14,000 g for 10 min. The supernatant was centrifuged at 100,000 g for 60 min to separate the membrane fraction (pellet) from the cytosolic fraction (supernatant). PLA2 activity was assessed by incubating enzyme (200 μg of cellular protein or 8 μg of membrane protein) with 100 μM (16:0, [3H]18:1) plasmenylcholine, (16:0, [3H]20:4) plasmenylcholine, (16:0, [3H]18:1) phosphatidylcholine, or (16:0, [3H]20:4) phosphatidylcholine (specific activity ∼150 dpm/pmol) in assay buffer containing 10 mM Tris, 10% glycerol, 4 mM EGTA, pH 7.0, at 37°C for 5 min in a total volume of 200 μl. For determining the sensitivity of membrane activity to R-BEL or S-BEL, the appropriate inhibitor was added to the isolated membrane fraction in the assay buffer for 10 min before the initiation of the activity assay. Reactions were initiated by adding the radiolabeled phospholipid substrate as a concentrated stock solution in ethanol. Reactions were terminated by the addition of 100 μl of butanol. The radiolabeled fatty acid released in the above reaction was isolated by application of 25 μl of the butanol phase to channeled Silica Gel G plates and then developed in petroleum ether-diethyl ether-acetic acid (70:30:1 vol/vol/vol). Results were quantified by liquid scintillation spectrometry.
Arachidonic acid release.
HSAEC were grown to confluence in 35-mm tissue culture dishes. Arachidonic acid release was determined by measuring [3H]arachidonic acid released into the surrounding medium from HSAEC prelabeled with 1 μCi of [3H]arachidonic acid (specific activity 100 Ci/mmol; PerkinElmer Life Sciences, Boston, MA) per culture dish for 18 h. Under these incubation conditions, 88% of the radiolabel was incorporated into the ethanolamine phospholipid pools of HSAEC. Analysis of the localization of incorporated exogenous deuterated arachidonic acid by mass spectrometry identified deuterated arachidonic acid in (16:0, 20:4), (18:0, 20:4), and (18:1, 20:4) plasmenylethanolamine, (16:0, 20:4) and (18:0, 20:4) phosphatidylethanolamine, and (16:0, 20:4) alkylacyl glycerophosphoethanolamine. Cells were washed three times with HEPES buffer containing, in mmol/l, 133.5 NaCl, 4.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 1.2 KH2PO4, 10 HEPES (pH 7.4), 10 glucose, and 0.36% BSA and incubated at 37°C for 15 min before experimental conditions. At the end of the stimulation period, the surrounding medium was transferred to a scintillation vial, the remaining cells were lysed in 10% SDS, and the lysate was then transferred to a separate vial. Radioactivity in the medium and cells was quantified by liquid scintillation spectrometry. Arachidonic acid mobilized from cellular phospholipids was expressed as the percentage of total incorporated radioactivity.
PGE2 release.
HSAEC were grown to confluence in 16-mm tissue culture dishes. Cells were washed twice with HBSS containing, in mmol/l, 135 NaCl, 0.8 MgSO4, 10 HEPES (pH 7.6), 1.2 CaCl2, 5.4 KCl, 0.4 KH2PO4, 0.3 Na2HPO4, and 6.6 glucose. After washing, 0.5 ml of HBSS with 0.36% BSA was added to each culture well. HSAEC were then stimulated with the appropriate tryptase concentrations. The surrounding buffer was removed from the HSAEC after selected time intervals, and PGE2 release was measured immediately using an immunoassay kit (R&D Systems, Minneapolis, MN). The protein content for the HSAEC confluent monolayers was determined in three representative cell culture wells and was assumed to be constant between wells for each experiment.
PAF assay.
HSAEC grown in 34-mm culture dishes (Corning) were washed twice with HBSS. Cells were incubated with 10 μCi of [3H]acetic acid per well for 20 min. After stimulation with tryptase, lipids were extracted from the cells using the method of Bligh and Dyer. The chloroform layer was concentrated by evaporation under nitrogen, resuspended in 9:1 CHCl3-CH3OH, applied to a silica gel 60 TLC plate, and developed in chloroform-methanol-acetic acid-water (50:25:8:4 vol/vol/vol/vol). The region corresponding to [3H]PAF was scraped, and radioactivity was quantified by liquid scintillation spectrometry. Loss of PAF during extraction and chromatography was corrected by adding a known amount of [14C]PAF as an internal standard.
PAF-acetylhydrolase assay.
HSAEC grown to confluence were incubated with BEL for 10 min and then removed from the tissue culture plate in 1.2 mM Ca2+ HEPES buffer and sonicated on ice. Cellular protein (25 μg) was incubated with 0.1 mM [acetyl-3H]PAF (10 mCi/mmol) for 30 min at 37°C. The reaction was stopped by the addition of acetic acid and sodium acetate. Released [3H]acetic acid was isolated by passing the reaction mixture through a C18 silica gel column (J. T. Baker, Phillipsburg, NJ), and eluted radioactivity was measured using a liquid scintillation counter.
Neutrophil isolation procedure.
Adult peripheral blood was collected (approved by Saint Louis University School of Medicine Institutional Review Board no. 12369) in vials containing 3.8% sodium citrate layered over Polymorphprep (Axis-Shield PoC AS, Oslo, Norway) and centrifuged at 500 g for 30 min. The top band at the sample/medium interface consisting of mononuclear cells and the lower band of polymorphonuclear cells were removed and washed with HBSS. Supernatant was discarded, and the cell pellet resuspended with 3 ml of 0.2% NaCl and incubated for 3 min at room temperature to lyse the red cells. Cells were again resuspended in HBSS and centrifuged at 175 g for 10 min at 4°C. Supernatant was removed, and cells resuspended in 5 ml of ice-cold HBSS. An aliquot was taken for a cell count using a hemacytometer.
Neutrophil adherence assay.
Neutrophils were resuspended in HBSS at 1 × 106 cells per milliliter. HSAEC were grown to confluence on a 12-well plate and washed with HBSS, and respective stimulants/inhibitors were added. CV3988 was added directly to the neutrophils when used. Five hundred microliters of neutrophils in suspension were added to each of the wells and incubated for 10 min at room temperature. Media and unbound neutrophils were removed and discarded. Plates were washed twice with Dulbecco's PBS. One milliliter of 0.2% Triton X-100 was added to each well to lyse adherent neutrophils and HSAEC. Cell lysates were scraped from the plate and transferred to an Eppendorf tube. A 500-μl aliquot of neutrophil suspension was added to 500 μl of 0.2% Triton X-100 and used as the theoretical maximal binding sample. Samples were sonicated (550 Sonic Dismembrator; Fisher Scientific, Pittsburgh, PA) for 10 s. To measure neutrophil peroxidase activity, 400 μl of cell lysate was transferred to a glass tube, 1 ml of PBS, 1.2 ml of HBSS + BSA, 200 μl of 3,3′-dimethoxybenzidine, and 200 μl of 0.05% H2O2 were added, and the mix was incubated for 15 min at room temperature. Two hundred microliters of 1% sodium azide (NaN3) was added to stop the reaction. The absorbance was then measured using a 4050 UV-Visible Spectrophotometer (Biochrom, Cambridge, England) at 450 nm.
Statistical analysis.
Data were analyzed using the Student's t-test. P values of <0.05 were considered statistically significant; P values of <0.01 were considered highly statistically significant. ANOVA was used for comparison between multiple groups.
RESULTS
Effect of tryptase on PLA2 activity.
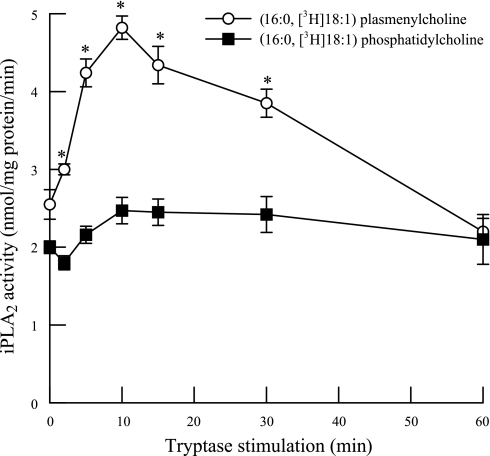
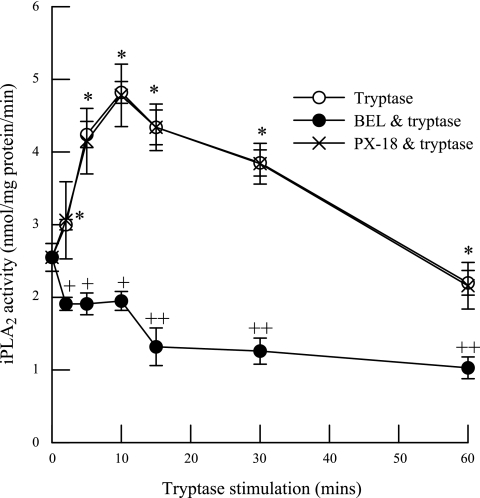
In initial experiments, we measured PLA2 activity in membrane and cytosolic subcellular fractions from HSAEC using (16:0, [3H]18:1) or (16:0, [3H]20:4) plasmenylcholine or phosphatidylcholine substrates in the presence (1 mM) or absence (4 mM EGTA) of calcium (Table 1). The majority of HSAEC PLA2 activity was measured in the absence of calcium (iPLA2), membrane-associated, and selective for arachidonylated (16:0, [3H]20:4) phospholipids (Table 1). Tryptase stimulation of HSAEC iPLA2 activity was significantly increased after 2 min and remained elevated for up to 30 min stimulation when activity was measured using (16:0, [3H]18:1) plasmenylcholine substrate in the absence of calcium (Fig. 1, open circles). However, no significant increase in iPLA2 activity was observed in tryptase-stimulated HSAEC when activity was measured using (16:0, [3H]18:1) phosphatidylcholine substrate (Fig. 1, closed squares). These data are consistent with our (35) previous finding in endothelial cells from other vascular beds and suggest that tryptase stimulates a plasmalogen-selective iPLA2. The tryptase-stimulated increase in iPLA2 activity was completely inhibited by pretreating the cells with the iPLA2-selective inhibitor BEL (2 μM, 10 min; Fig. 2, closed circles) but not by the secretory PLA2 (sPLA2) inhibitor PX-18 (2 μM, 10 min; Fig. 2, X). These data support our hypothesis that iPLA2 activity is increased in tryptase-stimulated HSAEC.
Table 1.
PLA2 activity (nmol·mg protein−1·min−1) in membrane and cytosolic subcellular fractions from HSAEC
| Cell Fraction | Substrate* | EGTA | Ca2+ |
|---|---|---|---|
| Membrane | Plasmenylcholine | ||
| 16:0, [3H]18:1 | 3.71±0.43 | 4.77±0.65 | |
| 16:0, [3H]20:4 | 6.23±0.63 | 8.48±0.52 | |
| Phosphatidylcholine | |||
| 16:0, [3H]18:1 | 3.16±0.29 | 3.63±0.35 | |
| 16:0, [3H]20:4 | 6.11±0.43 | 7.03±0.48 | |
| Cytosol | Plasmenylcholine | ||
| 16:0, [3H]18:1 | 0.28±0.17 | 0.41±0.17 | |
| 16:0, [3H]20:4 | 0.85±0.15 | 0.93±0.18 | |
| Phosphatidylcholine | |||
| 16:0, [3H]18:1 | 0.27±0.16 | 0.33±0.12 | |
| 16:0, [3H]20:4 | 0.77±0.12 | 0.72±0.18 |
Values represent means ± SE for separate measurements from 4 different cell isolations. PLA2 activity was measured in membrane and cytosolic subcellular fractions from human small airway epithelial cells (HSAEC) using plasmenylcholine or phosphatidylcholine substrates in the absence (4 mM EGTA) or presence (1 mM Ca2+) of calcium.
Substrate composition is represented as a:b, c:d where a:b and c:d represent the chain length: number of double bonds for the aliphatic groups at the sn-1 and sn-2 positions, respectively, of the corresponding phospholipid substrate molecule.
Fig. 1.
Activation of calcium-independent phospholipase A2 (iPLA2) in tryptase-stimulated human small airway epithelial cells (HSAEC). PLA2 activity was measured using 100 μM (16:0, [3H]18:1) plasmenylcholine (open circles) or (16:0, [3H]18:1) phosphatidylcholine (closed squares) in the absence of calcium (4 mM EGTA). Data represent means ± SE for 4 separate experiments. *P < 0.05.
Fig. 2.
Activation of iPLA2 following tryptase stimulation in HSAEC. iPLA2 activity was measured in tryptase (20 ng/ml)-treated HSAEC with or without pretreatment with bromoenol lactone (BEL; 2 μM, 10 min) or PX-18 (2 μM, 10 min). Data represent means ± SE for 4 separate experiments. *P < 0.05 compared with unstimulated cells, +P < 0.05 and ++P < 0.01 compared with BEL-pretreated cells.
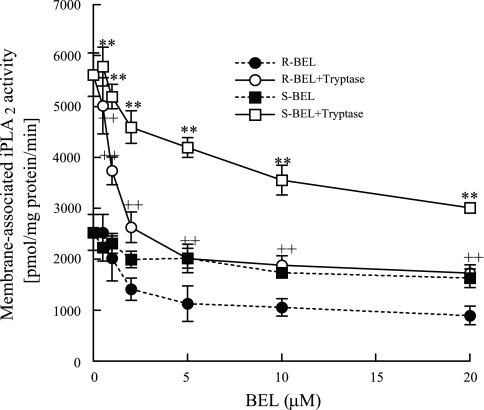
We further examined the specific iPLA2 isoform responsible for the increased activity measured in tryptase-stimulated HSAEC. We stimulated HSAEC with tryptase and then isolated the membrane and cytosolic subcellular fractions. iPLA2 activity was increased in the membrane fraction of tryptase-stimulated HSAEC, but cytosolic iPLA2 activity was not significantly altered (data not shown). Subsequently, the membrane fraction was incubated with increasing concentrations of R-BEL (selectively inhibits iPLA2γ) or S-BEL (selectively inhibits iPLA2β) for 10 min before the assay of iPLA2 activity. We found that iPLA2 activity was significantly inhibited by pretreatment with concentrations of R-BEL greater than 1 μM but was not inhibited by S-BEL until concentrations greater than 5 μM were used (Fig. 3). These data indicate that iPLA2γ is the major isoform present in HSAEC.
Fig. 3.
HSAEC were split into membrane and cytosol fractions. iPLA2 activity was measured in the presence of R-enantiomer BEL (R-BEL) or S-BEL with or without tryptase (20 ng/ml, 10 min). Results are representative of means ± SE of 4 separate experiments. **P < 0.01 for S-BEL and tryptase or ++P < 0.01 for R-BEL and tryptase treated cells compared with cells treated with R- or S-BEL only.
Effect of tryptase on arachidonic acid release from HSAEC.
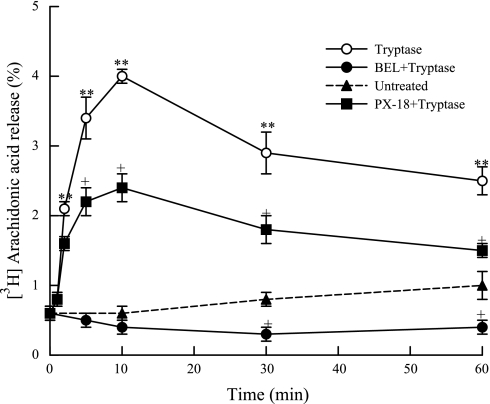
Tryptase stimulation results in a fourfold increase in arachidonic acid release by 10 min compared with unstimulated cells (Fig. 4). Pretreatment with BEL (2 μM, 10 min) before tryptase stimulation completely inhibited tryptase-induced iPLA2 activation (Fig. 2) and arachidonic acid release (Fig. 4). Pretreatment of HSAEC with PX-18 (2 μM, 10 min) did not inhibit iPLA2 activity (Fig. 2) but inhibited tryptase-stimulated arachidonic acid release by ∼50% (Fig. 4). This indicates that arachidonic acid release in tryptase-stimulated HSAEC is dependent on activation of iPLA2 but that sPLA2 is also involved in tryptase-stimulated arachidonic acid release.
Fig. 4.
Arachidonic acid release was measured from tryptase (20 ng/ml, 10 min)-stimulated HSAEC with or without pretreatment with BEL (2 μM, 10 min) or with PX-18 (2 μM, 10 min). Results are representative of means ± SE of 3 independent experiments. **P < 0.01 compared with unstimulated cells. +P < 0.05 compared with tryptase-treated cells.
Effect of tryptase on PGE2 release in the presence of PLA2 inhibitors.
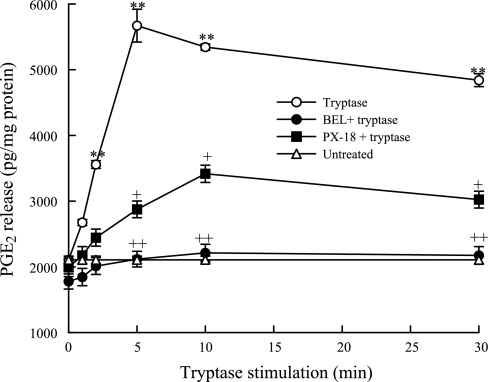
We stimulated HSAEC with tryptase and measured PGE2 release in the presence and absence of PLA2 inhibitors. Tryptase stimulation of HSAEC resulted in a significant increase in PGE2 release after 2 min (Fig. 5). Pretreatment with BEL (2 μM, 10 min) before tryptase stimulation completely inhibited the PGE2 release. Pretreatment with PX-18 (2 μM, 10 min) inhibited the tryptase-induced PGE2 release by ∼70% in these cells (Fig. 5).
Fig. 5.
PGE2 release was measured from tryptase (20 ng/ml)-stimulated HSAEC with or without pretreatment with BEL (2 μM, 10 min) or PX-18 (2 μM, 10 min). Cells were also left untreated. Results are representative of means ± SE of 3 independent experiments. **P < 0.01 compared with untreated cells. +P < 0.05 and ++P < 0.01 compared with tryptase-treated cells.
Effect of tryptase on PGE2 release in the presence of cyclooxygenase inhibitors.
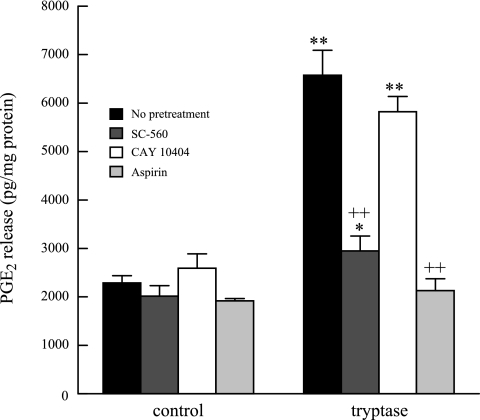
Arachidonic acid is metabolized by cyclooxygenase (COX)-1 and COX-2 to generate PGE2 in epithelial cells. Pretreatment with SC-560 (15 nM, 10 min, COX-1-selective inhibitor) or aspirin (1.5 nM, 10 min, COX-1 and COX-2 inhibitor) completely inhibited tryptase-stimulated PGE2 release from HSAEC (Fig. 6). However, pretreatment of cells with CAY10404 (1 nM, 10 min, a COX-2-selective inhibitor) had no effect. These data indicate that in the immediate phase of tryptase-stimulated PGE2 production it is the constitutive COX-1 isoform that is responsible for metabolizing iPLA2-catalyzed arachidonic acid release to generate PGE2.
Fig. 6.
HSAEC were stimulated with tryptase for 10 min (black bars). PGE2 release was measured with and without pretreatment with SC-560 (15 nM, 10 min), CAY10404 (1 nM, 10 min), and aspirin (1.5 mM, 10 min). Results represent means ± SE of 4 independent experiments samples *P < 0.05, **P < 0.01 compared with unstimulated cells. ++P < 0.01 compared with tryptase-stimulated cells.
Effect of tryptase on PAF production.
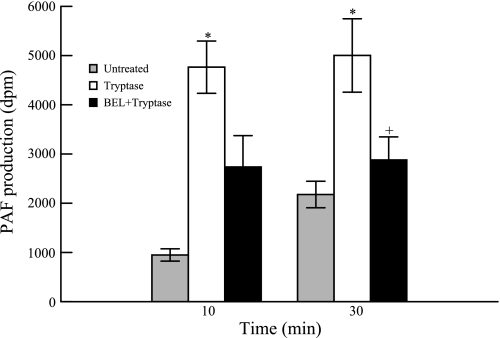
The lysophospholipid generated as a consequence of removal of sn-2 fatty acid from the phospholipid by iPLA2 is rapidly acetylated to PAF. PAF is a potent proinflammatory lipid metabolite that can result in disruption of the airway epithelial defense barrier. Tryptase stimulation of HSAEC caused an increase in PAF production that was observed until 30 min. This effect was attenuated by pretreating the cells with BEL (Fig. 7) indicating that tryptase-induced PAF production in HSAEC is at least in part due to iPLA2 activation. Treatment of HSAEC with concentrations of BEL up to 10 μM did not inhibit PAF-acetylhydrolase activity (5.72 nmol·mg protein−1·min−1 in untreated cells vs. 6.03 nmol·mg protein−1·min−1 in cells treated with 10 μM BEL for 10 min, n = 3 separate cell cultures).
Fig. 7.
Platelet activating-factor (PAF) production was measured in tryptase (20 ng/ml)-stimulated HSAEC at 10 and 30 min with or without pretreatment with BEL (2 μM, 10 min). Results represent means ± SE of 4 independent experiments. *P < 0.05 compared with unstimulated cells, +P < 0.05 compared with tryptase-stimulated cells.
Effect of tryptase on neutrophil adherence.
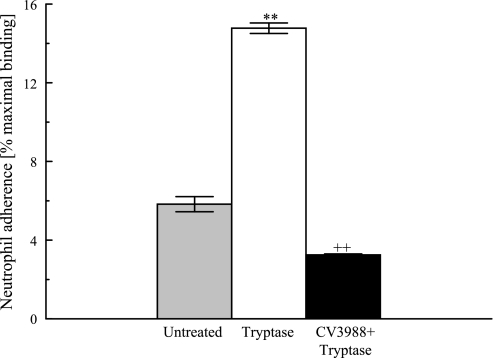
Previous studies from our laboratory have demonstrated significant increases in neutrophil adherence to tryptase-stimulated coronary artery endothelial cells. We examined whether tryptase stimulation would have a similar effect on the adherence of neutrophils to the HSAEC monolayer. Tryptase stimulation (20 ng/ml, 10 min) led to a significant increase in neutrophil adherence (Fig. 8). We also saw that pretreatment of neutrophils with a PAF receptor antagonist, CV3988, reduced the percentage of adherent neutrophils by 80% (from 14.77% ± 0.27% to 3.25% ± 0.25%, n = 4) demonstrating the importance of PAF-PAF receptor interactions in the adherence of these cells.
Fig. 8.
HSAEC were stimulated with tryptase (20 ng/ml) for 10 min with or without pretreatment with CV3988 (10 μM, 10 min). Neutrophils were added to the wells for 10 min, and peroxidase assay was done. Results represent means ± SE of 4 separate samples. **P < 0.01 compared with unstimulated cells, ++P < 0.01 compared with tryptase-stimulated cells.
DISCUSSION
Activation of mast cells can release several mediators from their granules that are responsible for a variety of inflammatory allergic disorders such as allergic rhinitis, asthma, dermatitis, and anaphylactic responses. In the lung, mast cells are found widely distributed in pleura, peribronchial regions, alveolar septa adjacent to nerves and blood vessels, upper and lower respiratory epithelium, and within the bronchial lumen (8). Tryptase released from mast cell granules has widespread effects on cellular proteins that can contribute to the pathogenesis of pulmonary inflammation in large and small airways (41). Tryptase degrades vasoactive intestinal polypeptide, an endogenous bronchodilator, generates bronchoconstrictor kinins, and activates prekallikrein (9). It also increases airway smooth muscle responsiveness to histamine in vitro and airway hyper responsiveness in vivo.
Airway inflammation in asthma is widely considered to be due to changes in the large airways. However, there is a significant patient population that does not respond to currently available antiasthmatic therapeutic regimens. In such cases, it is believed that the small airway (<2 mm in diameter) could be responsible for the residual inflammation. However, there are technical difficulties associated with evaluation of distal lung tissue. Only a small number of studies, using tissue obtained at the time of tumor resection or alveolar tissue from transbronchial biopsies, have evaluated distal lung inflammatory cell distribution and function (31, 38, 40).
Small airways consist of the respiratory bronchiole and terminal bronchioles that are devoid of cartilage and mucous secreting glands (12). Pathological changes in the small airways may proceed long before clinical symptoms of pulmonary inflammation become apparent. A hallmark of airway inflammation is widespread damage to the epithelium. There is infiltration by neutrophils and activated eosinophils (23). Epithelial sloughing occurs during exacerbation of bronchial asthma and is due to a possible defect in adherence to the basement membrane (5). The degree of airway hyperresponsiveness has been postulated to correlate with the loss of airway epithelial cells (45).
The airway epithelium can serve as both a target and effector cell in propagating the inflammatory response. On encountering allergens, the airway epithelium reacts by increasing mucus secretion, ciliary beat frequency, and changes in the ion transport/barrier function. The inflammatory metabolites released by the epithelium can act in an autocrine or paracrine fashion to exert widespread effects. Previous studies have demonstrated that airway epithelium can release IFN-γ and TNF-α. These in turn can lead to production of several secondary mediators such as prostanoids (PGE2), nonprostanoid mediators including epithelium derived relaxant/inhibitory factor, reactive oxygen species, and nitric oxide. PGE2 can have both pro- and anti-inflammatory effects. Inhaled PGE2 abolishes exercise-induced bronchoconstriction (29). It also inhibits bronchial hyperresponsiveness to inhaled allergen and attenuates antigen-induced eosinophilic inflammation (11, 33). Since PGE2 is a potent vasodilator, it potentiates the edema induced by other mediators (46). In addition, it reinforces the T helper type 2 (TH2) responses by increasing T cell polarization and release of IL-10 while inhibiting the release of IL-12 (a TH1-polarizing cytokine) (13).
To the best of our knowledge, this is the first study that demonstrates that mast cell tryptase can activate membrane-associated iPLA2γ in small airway epithelial cells resulting in arachidonic acid release, which is metabolized by COX-1 to generate PGE2. Pretreating the cells with BEL led to complete inhibition of these responses, whereas PX-18 led to partial inhibition of arachidonic acid release and PGE2 generation. This suggests that iPLA2 is required for the production of arachidonic acid in the immediate response to tryptase in airway epithelial cells but that sPLA2 may also be involved to a lesser degree.
There are three main classes of PLA2 that coexist in mammalian cells, secretory, cytosolic, and calcium-independent PLA2. They are activated in a temporal sequence and exert their effect over varying lengths of time depending on the stimulus and cell type (30). The cyclooxygenases convert arachidonic acid to an intermediate precursor PGH2. The release of arachidonic acid and formation of PGH2 is the crucial rate-limiting step for prostaglandin synthesis. In the early phase of prostaglandin biosynthetic response, increased arachidonic acid production by iPLA2 activation is followed by oxidation by constitutive COX-1 and prostaglandin biosynthesis (32).
Several published studies have described a possible role for iPLA2 in the generation of eicosanoids. IgG receptors (FcɛR) have been demonstrated to functionally couple to iPLA2β for the release of arachidonic acid and the production of leukotriene B4 and PGE2 (43). Murakami et al. (32) transfected various PLA2 and COX enzymes into human embryonic kidney cells and observed that ionophore-induced immediate PGE2 generation was linked to iPLA2β and COX-1 activity. They suggested that iPLA2 releases arachidonic acid in closer proximity to COX-1 than COX-2 and that iPLA2-derived arachidonic acid is somehow inaccessible to COX-2 (35). We report that immediate release of PGE2 from tryptase-stimulated HSAEC is dependant on activation of membrane-associated iPLA2γ and subsequent metabolism of arachidonic acid by COX-1, further supporting this hypothesis.
Although intracellular PLA2 isoforms may be directly coupled to COX in the cell for eicosanoid production, several studies suggest that immediate eicosanoid production involves cytosolic PLA2 (cPLA2) and sPLA2 with cPLA2 being the activator of the response but sPLA2 providing the bulk of arachidonic acid (22). Since tryptase-stimulated PGE2 production in HSAEC was significantly inhibited by pretreatment with BEL and partly by PX-18, it is possible that a similar interaction between iPLA2 and sPLA2 is involved in arachidonic acid release. Cross talk between cPLA2 and sPLA2 has been previously demonstrated in P388D1 macrophages in which intracellular arachidonic acid release by cPLA2 regulates the accessibility of sPLA2 to its substrate in the membrane (3). A similar mechanism, at least in part, may exist between iPLA2 and sPLA2 in HSAEC since PX-18 inhibits tryptase-stimulated arachidonic acid release and PGE2 production but does not inhibit iPLA2 activity.
In our studies, we also saw increased adherence of neutrophils to tryptase-stimulated HSAEC mediated by PAF. Holtzman et al. (16) have previously demonstrated PAF production by airway epithelial cells. PAF activates leukocytes and platelets via specific cell surface receptors, induces leukocyte chemotaxis, and stimulates preferential migration of eosinophils into the airway (2). It can also induce airway smooth muscle contraction and hyperreactivity in healthy subjects (19). Airway epithelial cells express several leukocyte adhesion molecules, and the receptors for these ligands are regulated by PAF (24, 44).
Infiltration and adherence of neutrophils occurs during asthma and chronic bronchitis and is also a hallmark of cystic fibrosis (39). Tryptase is known to have mitogenic effect on airway smooth muscles. In conjunction with increased neutrophil adherence, it may set up a cycle of inflammation that can induce airway remodeling and compromise lung function (7). In light of the close proximity of HSAEC and the interstitial mast cells, the effects of tryptase seen in the above-mentioned studies can be an important pathway in propagating small airway inflammation.
The epithelium is usually regarded as a target for inflammatory mediators, but this study demonstrates that it is capable of initiating and sustaining an inflammatory response. Once targeted, the epithelium sets a cascade of inflammatory events, which can be responsible for activation of inflammatory cells, stimulation of smooth muscle, and fibroblast proliferation. Molecular mechanisms that regulate COX and lipoxygenase production in airway epithelium provide an important therapeutic target. In asthma, PLA2 activation triggered by mast cell tryptase may contribute to the propagation of inflammation via the production of several membrane phospholipid-derived metabolites. Thus the development of a specific PLA2 inhibitor has the potential to be a valuable therapeutic tool for treating early airway inflammation that contributes further to the pathogenesis of asthma.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-68588 (J. McHowat) and the American Heart Association Heartland Affiliate 0610118Z (P. Rastogi).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem 270: 445–450, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Arnoux B, Denjean A, Page CP, Nolibe D, Morley J, Benveniste J. Accumulation of platelets and eosinophils in baboon lung after paf-acether challenge. Inhibition by ketotifen. Am Rev Respir Dis 137: 855–860, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Balsinde J, Bianco L, Ackermann EJ, Condefrieboes K, Dennis EA. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation phospholipid remodeling in P388D1 macrophages. Proc Natl Acad Sci USA 92: 8527–8531, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balsinde J, Dennis EA. Bromoenol lactone inhibits magnesium-dependent phosphatidate phosphohydrolase and blocks triacylglycerol biosynthesis in mouse P388D1 macrophages. J Biol Chem 271: 31937–31941, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Beasly R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthmatics. Am Rev Respir Dis 139: 806–817, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Beckett CS, Kell PJ, Creer MH, McHowat J. Phospholipase A2-catalyzed hydrolysis of plasmalogen phospholipids in thrombin-stimulated human platelets. Thromb Res 120: 259–268, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JK, Jones CA, Rooney LA, Caughey GH, Hall IP. Tryptase's potent mitogenic effects in human airway smooth muscle cells are via nonproteolytic actions. Am J Physiol Lung Cell Mol Physiol 282: L197–L206, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Carroll NG, Mutavdzic S, James AL. Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur Respir J 19: 879–885, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Caughey GH, Leidig F, Viro NF, Nadel JA. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther 244: 133–137, 1988. [PubMed] [Google Scholar]
- 10.Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in oxidant-induced renal cell death. Am J Physiol Renal Physiol 283: F492–F498, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Gauvreau GM, Watson RM, O'Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med 159: 31–36, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, Hegele RG, Hogg JC. Inflammation of small airways in asthma. J Allergy Clin Immunol 100: 44–51, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2 issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol 168: 2255–2263, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hazen SL, Stuppy RJ, Gross RW. Purification and characterization of canine myocardial cytosolic phospholipase A2. A calcium-independent phospholipase with absolute f1-2 regiospecificity for diradyl glycerophospholipids. J Biol Chem 265: 10622–10630, 1990. [PubMed] [Google Scholar]
- 15.Hazen SL, Zupan LA, Weiss RH, Getman DP, Gross RW. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. J Biol Chem 266: 7227–7232, 1991. [PubMed] [Google Scholar]
- 16.Holtzman MJ, Ferdman B, Bohrer A, Turk J. Synthesis of the 1-O-hexadecyl molecular species of platelet-activating factor by airway epithelial and vascular endothelial cells. Biochem Biophys Res Commun 177: 357–364, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Jeffery PK Histological features of the airways in asthma and COPD. Respiration 59: 13–16, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins CM, Han X, Mancuso DJ, Gross RW. Identification of calcium-independent phospholipase A2 (iPLA2) beta, and not iPLA2 gamma, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2s. J Biol Chem 277: 32807–32814, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kaye MD, Smith LJ. Effects of inhaled leukotriene D4 and platelet-activating factor on airway reactivity in normal subjects. Am Rev Respir Dis 141: 993–997, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Knight DA, Stewart GA, Thompson PJ. Prostaglandin E2, but not prostacyclin inhibits histamine-induced contraction of human bronchial smooth muscle. Eur J Pharmacol 272: 13–19, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca2+-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta 1302: 55–60, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Liu ES, Shin VY, Ye YN, Luo JC, Wu WK, Cho CH. Cyclooxygenase-2 in cancer cells and macrophages induces colon cancer cell growth by cigarette smoke extract. Eur J Pharmacol 518: 47–55, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Lommatzsch M, Julius P, Kuepper M, Garn H, Bratke K, Irmscher S, Luttmann W, Renz H, Braun A, Virchow JC. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J Allergy Clin Immunol 118: 91–97, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Look DC, Rapp SR, Keller BT, Holtzman MJ. Selective induction of intercellular adhesion molecule-1 by interferon-γ in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 263: L79–L87, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 81: 77–80, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J Biol Chem 275: 9937–9945, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso DJ, Jenkins CM, Sims HF, Cohen JM, Yang J, Gross RW. Complex transcriptional and translational regulation of iPLA2 γ resulting in multiple gene products containing dual competing sites for mitochondrial or peroxisomal localization. Eur J Biochem 271: 4709–4724, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Mancuso DJ, Han X, Jenkins CM, Lehman JJ, Sambandam N, Sims HF, Yang J, Yan W, Yang K, Green K, Abendschein DR, Saffitz JE, Gross RW. Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent phospholipase A2γ. J Biol Chem 282: 9216–9227, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Melillo E, Woolley KL, Manning PJ, Watson RM, O'Byrne PM. Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am J Respir Crit Care Med 149: 1138–1141, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Meyer MC, Rastogi P, Beckett CS, McHowat J. Phospholipase A2 inhibitors as potential anti-inflammatory agents. Curr Pharm Des 11: 1301–1312, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Minshall EM, Hogg JC, Hamid QA. Cytokine mRNA expression in asthma is not restricted to the large airways. J Allergy Clin Immunol 101: 386–390, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Murakami M, Kambe T, Shimbara S, Kudo I. Functional coupling between various phospholipase A2s and cyclooxygenases in the immediate and delayed prostaglandin biosynthetic pathways. J Biol Chem 274: 3103–3115, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Pavord ID, Wong CS, Williams J, Tattersfield AE. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am Rev Respir Dis 148: 87–90, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Platshon LF, Kaliner M. The effects of the immunologic release of histamine upon human lung cyclic nucleotide levels and prostaglandin generation. J Clin Invest 62: 1113–1121, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portell C, Rickard A, Vinson S, McHowat J. Prostacyclin production in tryptase and thrombin stimulated human bladder endothelial cells: effect of pretreatment with phospholipase A2 and cyclooxygenase inhibitors. J Urol 176: 1661–1665, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Rastogi P, Beckett CS, McHowat J. Prostaglandin production in human coronary artery endothelial cells is modulated differentially by selective phospholipase A2 inhibitors. Prostaglandins Leukot Essent Fatty Acids 76: 205–212, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761: 1246–1259, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland ER, Martin RJ, Bowler RP, Zhang Y, Rex MD, Kraft M. Physiologic correlates of distal lung inflammation in asthma. J Allergy Clin Immunol 113: 1046–1050, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Tabary O, Corvol H, Boncoeur E, Chadelat K, Fitting C, Cavaillon JM, Clément A, Jacquot J. Adherence of airway neutrophils and inflammatory response are increased in CF airway epithelial cell-neutrophil interactions. Am J Physiol Lung Cell Mol Physiol 290: L588–L596, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Taha RA, Minshall EM, Miotto D, Shimbara A, Luster A, Hogg JC, Hamid QA. Eotaxin and monocyte chemotactic protein-4 mRNA expression in small airways of asthmatic and nonasthmatic individuals. J Allergy Clin Immunol 103: 476–483, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Tam EK, Caughey GH. Degradation of airway neuropeptides by human lung tryptase. Am J Respir Cell Mol Biol 3: 27–32, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka H, Takeya R, Sumimoto H. A novel intracellular membrane-bound calcium-independent phospholipase A2. Biochem Biophys Res Commun 272: 320–326, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Tay HK, Melendez AJ. Fcgamma RI-triggered generation of arachidonic acid and eicosanoids requires iPLA2 but not cPLA2 in human monocytic cells. J Biol Chem 279: 22505–22513, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Tonnesen MG, Anderson DC, Springer TA, Knedler A, Avdi N, Henson PM. Adherence of neutrophils to cultured human microvascular endothelial cells. Stimulation by chemotactic peptides and lipid mediators and dependence upon the Mac-1, LFA-1, p150,95 glycoprotein family. J Clin Invest 83: 637–646, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis 137: 62–69, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Williams TJ Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br J Pharmacol 65: 517–524, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Han X, Gross RW. Identification of hepatic peroxisomal phospholipase A2 and characterization of arachidonic acid-containing choline glycerophospholipids in hepatic peroxisomes. FEBS Lett 546: 247–250, 2003. [DOI] [PubMed] [Google Scholar]