Abstract
Background and purpose:
Tetrazoles were recently developed as inhibitors of the cellular uptake of the endocannabinoid anandamide or of its hydrolysis by fatty acid amide hydrolase (FAAH), but were proposed to act also on non-endocannabinoid-related serine hydrolases.
Experimental approach:
We tested, in a model of inflammatory pain induced in mice by formalin, five chemically similar inhibitors: (i) OMDM119 and OMDM122, two potent carbamoyl tetrazole FAAH inhibitors with no effect on anandamide uptake; (ii) LY2183240, a carbamoyl tetrazole with activity as both FAAH and uptake inhibitor; (iii) OMDM132, a non-carbamoyl tetrazole with activity only as uptake inhibitor and iv) OMDM133, a non-carbamoyl tetrazole with no activity at either FAAH or uptake.
Results:
All compounds (2.5–10 mg kg−1, i.p.) inhibited the second phase of the nocifensive response induced by intraplantar injection of formalin. The effects of OMDM119, OMDM122 and OMDM133 were not antagonized by pretreatment with cannabinoid CB1 receptor antagonists, such as rimonabant or AM251 (1–3 mg kg−1, i.p.). The effects of LY2183240 and OMDM132 were fully or partially antagonized by rimonabant, respectively, and the latter compound was also partly antagonized by the CB2 receptor antagonist, AM630.
Conclusions and implications:
(i) non-FAAH hydrolases might be entirely responsible for the antinociceptive activity of some, but not all, tetrazole FAAH inhibitors, (ii) the presence of a carbamoylating group is neither necessary nor sufficient for such compounds to act through targets other than FAAH and (iii) inhibition of anandamide uptake is responsible for part of this antinociceptive activity, independently of effects on FAAH.
Keywords: endocannabinoid, FAAH, cannabinoid, anandamide, 2-arachidonoylglycerol, pain, uptake, inhibitor, tetrazole
Introduction
Recent studies have looked at the endocannabinoid system as a possible answer to the ever increasing demand for analgesic drugs, particularly against intractable conditions such as central and peripheral painful neuropathies and cancer-associated hyperalgesia (see Maione et al., 2006; Guindon et al., 2007). The direct targeting of cannabinoid CB1 receptors, which are very abundant in both brain and in peripheral nerve fibres, appears to be limited by the unavoidable psychotropic side effects but, judging from animal and clinical studies, an alternative approach might be to use CB1 agonists to enhance the antihyperalgesic effects of opiates and other analgesics (Maida et al., 2008), and to lower the levels of opiates necessary to achieve analgesia, thus reducing the chances of developing opiate tolerance, dependence and side effects (Smith et al., 2007; Narang et al., 2008). Furthermore, the use of Δ9-tetrahydrocannabinol together with a non-psychotropic cannabinoid, cannabidiol, seems to reduce the typical unwanted side effects of the former compound and increase the analgesic efficacy in both neuropathic pain from multiple sclerosis and cancer pain (Russo and Guy, 2006; Iskedjian et al., 2007; Rog et al., 2007). On the other hand, the selective targeting of cannabinoid CB2 receptors, which are much less abundant than CB1 receptors in the normal brain and not coupled to psychotropic effects, is being pursued by pharmaceutical companies, with many compounds in preclinical studies and one (GW842166X) in phase II clinical trials (Giblin et al., 2007; Jhaveri et al., 2007; Guindon and Hohmann, 2008; Kikuchi et al., 2008; Yao et al., 2008).
As selective activation of CB2 receptors might also have side effects, particularly in immune-compromised subjects, because of its actions on the immune system, several groups are trying to develop analgesic drugs from inhibitors of endocannabinoid degradation. During pathological conditions, the local concentrations of endocannabinoids are supposed to be altered, especially in those tissues affected by the condition, to provide analgesia, among other beneficial effects. (Di Marzo, 2008). Therefore, inhibitors of endocannabinoid degradation, such as blockers of endocannabinoid cellular reuptake and hydrolysis by fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase should mostly act in those tissues where endocannabinoids are being produced, released and degraded, thus potentially providing selective antihyperalgesic effects (Lichtman et al., 2004a; Chang et al., 2006; Jayamanne et al., 2006; La Rana et al., 2006; Comelli et al., 2007; Hohmann, 2007; Jhaveri et al., 2007; Maione et al., 2007; Mitchell et al., 2007; Russo et al., 2007), although not without potential complications (Di Marzo, 2008).
Recently, tetrazole-based compounds, based on LY2183240, have been developed as inhibitors of endocannabinoid reuptake or FAAH or both (Moore et al., 2005; Dickason-Chesterfield et al., 2006; Ortar et al., 2008a). However, based on the observation that most of these compounds contain a carbamoyl moiety capable of binding to activated serine residues present in a variety of hydrolases, the selectivity of these compounds has been questioned (Alexander and Cravatt, 2006). Proteomics-based methods have been used to show that compounds like LY2183240 do indeed bind to several different serine hydrolases (Alexander and Cravatt, 2006), although it has also been reported that their selectivity can be improved by appropriate chemical modifications (Ortar et al., 2008a). We have very recently reported that tetrazole-based compounds without the carbamoyl moiety typical of LY2183240, can still inhibit anandamide cellular uptake, without affecting FAAH and several other serine hydrolases involved in endocannabinoid level regulation (Ortar et al., 2008b). Nevertheless, although the analgesic activity of LY2183240 was previously described (Moore et al., 2005), no systematic study has been carried out so far to assess whether the antinociceptive effects of tetrazole-based inhibitors of anandamide inactivation is due to their inhibition of the cellular uptake process or FAAH, or both, or neither of these targets. For example, no study has been performed to understand whether compounds like LY2183240 cause analgesia because of inhibition of anandamide cellular uptake, FAAH or non-FAAH serine hydrolases. For this compound, which, when given i.p., dose-dependently attenuates formalin-induced paw-licking pain behaviour in the formalin model of persistent pain (Moore et al., 2005), it is not even known whether its actions are sensitive to CB1 receptor antagonists as with other inhibitors of endocannabinoid inactivation. In this study, we have aimed at filling these gaps by assessing the effects, in the formalin test in mice, of five different tetrazole-based inhibitors of endocannabinoid inactivation developed in previous studies (Moore et al., 2005; Ortar et al., 2008a, 2008b; Table 1), that is, (i) two carbamoyl tetrazoles with high potency at FAAH and no effect on anandamide uptake (OMDM119 and OMDM122); (ii) a carbamoyl tetrazole with activity as both FAAH and uptake inhibitor (LY2183240); (iii) a non-carbamoyl tetrazole with activity only as uptake inhibitor (OMDM132) and (iv) a non-carbamoyl tetrazole with no activity at either FAAH or anandamide uptake (OMDM133). Importantly, none of these compounds exhibits appreciable affinity for either CB1 or CB2 receptors (Moore et al., 2005; Ortar et al., 2008b; V Di Marzo, unpublished data), thus allowing us to rule out the possibility that any of their actions in vivo could be due to direct activation of these receptors. For each compound we have also assessed whether the effects observed were counteracted by CB1 receptor antagonists.
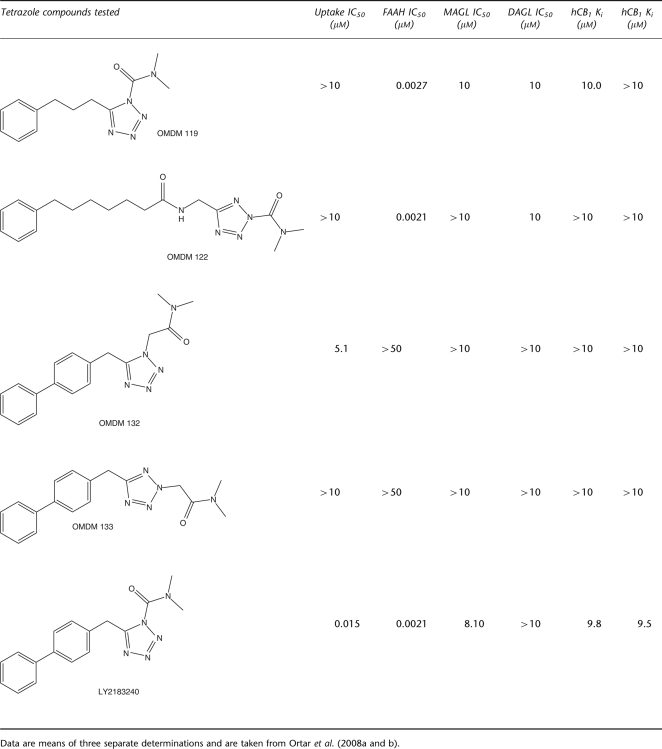
Table 1.
Effect of the five tetrazoles tested in this study on [14C] anandamide uptake by RBL-2H3 cells, [14C]Anandamide hydrolysis by rat brain membranes (fatty acid amide hydrolase (FAAH) assay), sn-1-[14C]oleoyl-2-arachidonoylglycerol conversion into 2-AG by COS cell membrane fractions overexpressing human recombinant diacylglycerol lipase-α (DAGL assay) and [3H]2-arachidonoylglycerol hydrolysis by COS cell cytosolic fractions (monoacylglycerol lipase (MAGL) assay)
Materials and methods
Formalin test
Animal care and all animal procedures were in compliance with Italian (D. L. 116/92) and EEC (O.J. of EC L358/1 18/12/1986) regulations on the protection of laboratory animals. Guidelines of the International Association for the Study of Pain were also followed. Male Swiss–Webster mice (40–45 g) were used in these experiments; they were housed at constant temperature (21±1 °C) and relative humidity (60%) under a regular light/dark schedule (light 7.00–19.00). Food and water were always available.
Formalin injection induces a biphasic typical nocifensive behaviour (Dubuisson and Dennis, 1977). Nociceptive responses are divided into an early, short lasting first phase (0–7 min) caused by a primary afferent discharge produced by the stimulus, followed by a quiescent period and then a second, prolonged phase (15–60 min) of tonic pain. Mice received formalin (1.25% in saline, 30 μL) in the dorsal surface of one side of the hindpaw. Each mouse was randomly assigned to one of the experimental groups and placed in a Plexiglas cage and allowed to move freely for 15–20 min. A mirror was placed at a 45° angle under the cage to allow full view of the hindpaws. Lifting, favouring, licking, shaking and flinching of the injected paw were recorded as nociceptive responses. These were measured every 5 min and expressed as their total duration in min (mean±s.e.mean) within each of the 5 min bins. The same observer, unaware of the treatments, scored all behavioural responses. Groups of 8–10 animals per treatment were used with each animal being used for one treatment only.
Recording of nocifensive behaviour commenced immediately after formalin injection and was continued for 60 min. The version of the formalin test that we used (and employed also in Maione et al., 2007) is based on the fact that correlational analyses showed that no single behavioural measure can be a strong predictor of formalin or drug concentrations on spontaneous behaviours (Abbott et al., 1995; Saddi and Abbott, 2000). Therefore, we considered that a simple sum of time spent licking and elevating the paw, or the weighted pain score (Dubuisson and Dennis, 1977) was better than any single behavioural measure (lifting, favouring, licking, shaking and flinching; r ranging from 0.75 to 0.86; see Abbott et al., 1995). Mice received, by intraperitoneal injection, vehicle (10% dimethylsulphoxide in 0.9% NaCl) or different doses of OMDM 119 (1–5 mg kg−1.), OMDM 122 (1–5 mg kg−1), OMDM 133 (1, 2.5 and 5 mg kg−1), LY2183240 (2.5–5 mg kg−1) or OMDM 132 (2.5–5 and 10 mg kg−1) alone or in combination with the selective cannabinoid CB1 receptor antagonists, rimonabant (1–2.5 mg kg−1) or AM251 (1–3 mg kg−1), the selective cannabinoid CB2 receptor antagonist, AM630 (1 mg kg−1) and administered 15 min before peripheral injection of formalin. The CB1 or CB2 receptor antagonists were administered 5 min before the test compounds.
Statistical analysis
Data are shown as means±s.e.mean from groups of 8–10 animals. Significant differences between group means were assessed by one-way ANOVA followed by Bonferroni's test.
Compounds
OMDM119, OMDM122, OMDM132, OMDM133 and LY2183240 (Table 1) were synthesized as described previously (Ortar et al., 2008a, 2008b). Their activity on human recombinant cannabinoid CB1 and CB2 receptors, anandamide cellular uptake, FAAH and other endocannabinoid-related serine hydrolases in vitro was established in previous studies (Ortar et al., 2008a and 2008b; V Di Marzo, unpublished data). All other drug/molecular target nomenclature used here conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2008).
Results
Effect of the five compounds tested on the two phases of formalin-induced nocifensive response in mice
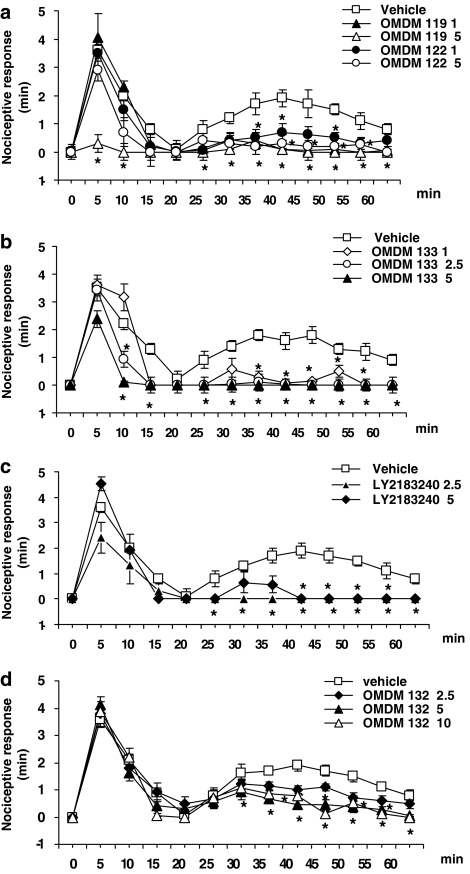
The dose-dependent effects of the five compounds (the in vitro pharmacology of these compounds is summarized in Table 1) on the two phases of formalin-induced nocifensive response are shown in Figure 1. OMDM119 completely blocked the second phase already at the lowest dose tested (1 mg kg−1), whereas it blocked also the first phase at the highest dose tested (5 mg kg−1) and at 5 min from injection (Figure 1a; P<0.05). In contrast, OMDM122, produced a strong, although not complete, effect on the second phase also at the lowest dose tested (1 mg kg−1; P<0.05), whereas it did not significantly affect the first phase even at the highest dose tested (5 mg kg−1; Figure 1a). OMDM133 was also maximally active on the second phase at the lowest dose tested (1 mg kg−1; P<0.05) and did not significantly affect the first phase even at the highest dose tested (5 mg kg−1) at 5 min from injection, although it did so at 10 and 15 min (Figure 1b). LY2183240 was also maximally active on the second phase at the lowest dose tested (2.5 mg kg−1; P<0.05) and did not significantly affect the first phase even at the highest dose tested (5 mg kg−1; Figure 1c). Finally, OMDM132 (a) produced a significant, although incomplete, effect on the second phase even at the highest dose tested (10 mg kg−1; which was the same as that exerted by the intermediate dose of 5 mg kg−1); (b) produced a nearly half-maximal response on this phase at the lowest dose tested (2.5 mg kg−1; P<0.05) and (c) did not significantly affect the first phase at any of the doses tested (Figure 1d). No overt behavioural changes were observed in this study following the administration of vehicle and of all the drugs at the doses used. Mice remained alert and generally active throughout the experiments.
Figure 1.
Antinociceptive effect of vehicle (10% dimethylsulphoxide in 0.9% NaCl) or OMDM119 and OMDM 122 (a), OMDM133 (b), LY2183240 (c) and OMDM 132 (d) in the formalin test in mice. Numbers after the names of the compounds indicate the doses (i.p.) used for each experiment. Each point represents the mean±s.e.mean of 8–10 animals per group. Data were analysed using one-way ANOVA followed by Bonferroni's test and statistical significance was taken as P<0.05. Asterisks denote statistically significant differences from vehicle.
Effect of cannabinoid receptor antagonists on the effects of the five compounds
Both FAAH inhibitors and blockers of endocannabinoid reuptake are known to inhibit the formalin-induced nocifensive response in a way that is usually antagonized only by CB1 receptor antagonists (La Rana et al., 2006; Maione et al., 2007; Sit et al. 2007). For this reason, we attempted to counteract the antinociceptive effects of the five compounds only with CB1 antagonists, except in the case of compounds, the effect of which responded to the CB1 antagonist, rimonabant, only in part. Rimonabant was used with all five compounds. In the case of some of those compounds the effect of which was clearly not antagonized by rimonabant, we also used the other CB1 antagonist, AM251, to rule out the possibility that the lack of antagonism was due to rimonabant-related pharmacokinetic reasons. The doses of compounds and antagonists for each antagonism experiments were chosen on the basis of the efficacy of the compounds per se in the formalin test, and were usually those being maximally active. The effects of OMDM119 and OMDM122 (1 and 5 mg kg−1) on the second phase of the formalin response were not antagonized by rimonabant (2.5 mg kg−1) nor, in the case of OMDM119, by AM251 (1 and 3 mg kg−1; Figures 2a and b, and data not shown). However, the lowest dose of AM251 (1 mg kg−1) did antagonize the effect of OMDM119 on the first phase of the formalin response (Figure 2b). Likewise, rimonabant (1 mg kg−1) did not antagonize the effect of OMDM133 (1 mg kg−1) on the second phase (Figure 2c). The effect of OMDM133 (2.5 and 5 mg kg−1) on this phase was also not antagonized by AM251 (3 mg kg−1), which however reduced part of the response of OMDM133 (2.5 mg kg−1) on the first phase (Figure 2d). Rimonabant (1 mg kg−1) was, instead, very effective in counteracting the effect of LY2183240 (2.5 mg kg−1) on the second phase of the formalin response (Figure 2e). Finally, rimonabant (1 mg kg−1) reversed only the first part of the effect of OMDM132 (5 mg kg−1) on the second phase of the formalin response. For this reason, only for this compound, we also tested the CB2 receptor antagonist AM630 (1 mg kg−1), which again reversed only the first part of the effect of OMDM132 (5 mg kg−1; Figure 2f).
Figure 2.
Antinociceptive effect of vehicle (10% dimethylsulphoxide in 0.9% NaCl) or five test compounds alone or in combination with rimonabant (rimonab), or AM251 or AM630 in the formalin test in mice. Numbers after the names of the compounds indicate the doses (i.p.) used for each experiment. Each point represents the mean±s.e.mean of 8–10 animals per group. Data were analysed using one-way ANOVA followed by Bonferroni's test and statistical significance was taken as P<0.05. Circles denote statistically significant differences from the data obtained with a given compound in the absence of antagonists and, in the case of panel f refer to both rimonabant and AM630. (a) Effect of rimonabant (2.5 mg kg−1) on OMDM 119 and OMDM 122 (5 mg kg−1); (b) Effect of AM251 (1 and 3 mg kg−1) on OMDM 119 (5 mg kg−1); (c) Effect of rimonabant (1 mg kg−1) on OMDM 133 (1 mg kg−1); (d) Effect of AM251 (3 mg kg−1) on OMDM 133 (2.5 and 5 mg kg−1); (e) Effect of rimonabant (1 mg kg−1) on LY2183240 (2.5 mg kg−1); (f) Effect of rimonabant (1 mg kg−1) or AM630 (1 mg kg−1) on OMDM 132 (5 mg kg−1).
Discussion
In this study, we have investigated for the first time the mechanism of action of tetrazole-based inhibitors of endocannabinoid inactivation by comparing the activities in the formalin test, and their sensitivity to CB1 receptor antagonists, of five structurally similar carbamoyl- and non-carbamoyl-containing compounds (Table 1), previously shown to inhibit either FAAH or anandamide cellular reuptake or both, with no affinity for CB1 or CB2 receptors (Moore et al., 2005; Ortar et al., 2008a and 2008b; V Di Marzo, unpublished data). Although one of these carbamoyl-tetrazoles (LY2183240) had been previously shown to inhibit the nocifensive response in the formalin test (Moore et al., 2005) and shown to inhibit also several serine hydrolases (Alexander and Cravatt, 2006), none of the compounds of this study had ever been tested in the presence of cannabinoid receptor antagonists to confirm their mechanism of action as inhibitors of endocannabinoid degradation and, hence, as indirect activators of cannabinoid receptors. Bearing in mind that the existence of a specific mechanism for anandamide cellular uptake has not yet been characterized from a molecular point of view, and that several authors believe that this process is mostly mediated by FAAH-driven gradient of anandamide concentration across the plasma membrane (see Fowler, 2006), our present findings can be summarized as follows (Table 2): (i) OMDM119 and OMDM122, that is, two carbamoyl-tetrazoles that inhibit FAAH without inhibiting anandamide cellular uptake (Ortar et al., 2008a), potently inhibited the second phase of the formalin-induced nocifensive response in a way not affected by CB1 receptor antagonists; (ii) LY2183240, a carbamoyl-tetrazole that inhibits both FAAH and anandamide cellular uptake (Moore et al., 2005; Dickason-Chesterfield et al., 2006) as well as some other serine hydrolases (Alexander and Cravatt, 2006), inhibits the second phase of the formalin-induced response in a way entirely inhibited by CB1 receptor antagonism; (iii) OMDM132, a non-carbamoyl-containing tetrazole that inhibits anandamide cellular uptake without affecting FAAH (Ortar et al., 2008b), inhibits the second phase of the formalin-induced response in a way partly affected by both CB1 and CB2 receptor antagonists and (iv) OMDM133, the OMDM132 regioisomer that does not inhibit anandamide cellular reuptake nor FAAH, still potently inhibits the second phase of the formalin-induced response but in a way unaffected by CB1 receptor antagonists.
Table 2.
Summary of the effects of the five compounds tested in this study
| Effect on the first phase | Effect on the second phase | Effect counteracted by rimonabant | Effect counteracted by AM251 | Effect counteracted by AM630 | |
|---|---|---|---|---|---|
| OMDM119 | YES Partial inhibition only at the highest dose tested (5 mg kg−1) |
YES Full inhibition at both doses tested (1 and 5 mg kg−1) |
NO | YES Only the effect on the first phase and only with the lowest dose of the antagonist (1 mg kg−1) |
Not tested |
| OMDM122 | NO | YES Partial and full inhibition at the two doses tested (1 and 5 mg kg−1), respectively |
NO | Not tested | Not tested |
| OMDM132 | NO | YES Partial inhibition at all doses tested (2.5, 5 and 10 mg kg−1) |
YES Only in part |
Not tested | YES Only in part |
| OMDM133 | YES Dose-related partial inhibition at all doses tested (1, 2.5 and 5 mg kg−1) |
YES Partial inhibition at the lowest dose tested, full inhibition at the two highest ones (1, 2.5 and 5 mg kg−1) |
NO | YES Only the effect on the first phase, and only in part |
Not tested |
| LY2183240 | NO | Full inhibition at both doses tested (2.5 and 5 mg kg−1) | YES Full counteraction |
Not tested | Not tested |
These findings suggest that: (i) some (that is, OMDM119, OMDM122), but not all (that is, LY2183240), carbamoyl-tetrazole FAAH inhibitors exert their antinociceptive effects in the formalin test by targeting proteins other than FAAH; (ii) the presence of a carbamoylating group is neither necessary (see results with OMDM133) nor sufficient (see results with LY2183240) for tetrazole compounds to affect non-FAAH proteins and inhibit formalin-induced pain in a non-CB1-mediated manner and (iii) inhibition of anandamide uptake is responsible for part of this antinociceptive effect, independently of effects on FAAH and non-FAAH proteins, as shown by the results with OMDM132 and, possibly, LY2183240.
The finding of the fully CB1 receptor-dependent antinociceptive effect of LY2183240 in the formalin test is in agreement with previous studies carried out with other FAAH or anandamide uptake inhibitors, such as OL-135 (Lichtman et al., 2004a), BMS-1 (Sit et al., 2007), N-arachidonoylserotonin (Maione et al., 2007; three FAAH inhibitors) and AM404 and UCM707 (La Rana et al., 2006; two uptake inhibitors), most of which, in this test, act only through CB1 receptors. More importantly, our present findings suggest that, at least in terms of its activity in the formalin test in mice, LY2183240 acts by inhibiting endocannabinoid inactivation, and not through the other, non-endocannabinoid-related serine hydrolases that were found to be inhibited by this compound in vitro (Alexander and Cravatt, 2006). OMDM132 is the non-carbamoyl-analogue of LY2183240, and, probably due to the lack of a electrophilic chemical moiety capable of attacking activated serine residues in serine hydrolases, does not inhibit FAAH nor some of the other enzymes that are inhibited by LY2183240 (Ortar et al., 2008a, 2008b). We found here that the antinociceptive effects of OMDM132, unlike those of LY2183240, were not completely blocked by either a CB1 or a CB2 receptor antagonist. This finding, while confirming that the carbamoyl group is not uniquely nor necessarily responsible for non-cannabinoid receptor-mediated actions of tetrazole-based compounds might also suggest that OMDM132 acted in part by interacting with targets other than the putative specific mechanism mediating anandamide cellular uptake. This possibility is supported by the observation that OMDM132 regioisomer, OMDM133, was found here to be more potent at inhibiting formalin-induced pain and to exert this effect in a way totally unaffected by CB1 receptor antagonists. The observation that the effect of OMDM132 was partly reversed also by a CB2 antagonist is not totally unprecedented, as Borsani et al (2007) showed that also the inhibitory effects of AM404 in formalin-treated rats were partly mediated by CB2 receptors.
OMDM119 and OMDM122 are two carbamoyl-containing tetrazoles that, unlike LY2183240, have proven to be selective for FAAH vs other endocannabinoid-related serine hydrolases tested in our laboratory (Table 1; Ortar et al., 2008a). However, their selectivity towards serine hydrolases in general has never been examined with global proteomics-based screens. The disappointing finding that their potent analgesic effect in the formalin test was not antagonized by CB1 receptor antagonists might be explained with their possible interaction with endocannabinoid-unrelated serine hydrolases (Alexander and Cravatt, 2006), but also with non-serine hydrolase targets, as appears to be the case for OMDM133. The possible alternative explanation that other, non-cannabinoid receptor-active, FAAH substrates, such as palmitoylethanolamide or N-arachidonoylglycine (Cravatt and Lichtman, 2002), participate in the effects of these two compounds is not supported by previous findings with other FAAH inhibitors (see above), nor by the previous observation that decreased sensitivity of FAAH null mice to formalin-induced pain is fully reversed by a CB1, but not CB2, receptor antagonist (Lichtman et al., 2004b). However, our present data do not allow us to rule out that part of the effect of OMDM119 and OMDM122 might be indeed due to the elevation of the levels of cannabinoid receptor-inactive FAAH substrates.
Some of the compounds found here to exert their antinociceptive effects on the second phase of the formalin test through non-CB1 receptors, that is, OMDM133 and, particularly, OMDM119, also affected the first phase of the formalin test and did so in a way partly reverted by the CB1 receptor antagonists, AM251. This would suggest that these compounds affect the acute early phase of formalin-induced nocifensive behaviour in a way partly mediated by CB1 receptors. Although this might be the case for OMDM119, which inhibits FAAH, it is unlikely to hold true also for OMDM133, which in vitro is not capable of activating CB1 receptors either directly or indirectly (Table 1). At any rate, rimonabant, unlike AM251, did not appear to antagonize the effects of OMDM119 and OMDM133 on the first phase, which therefore is unlikely to be mediated by CB1. On the other hand, LY2183240 and OMDM132 (which did reduce the second phase of the formalin response through cannabinoid receptors), and OMDM122 did not affect the first phase. Previously developed selective FAAH inhibitors, like BMS-1 and OL-135 (as well as FAAH gene knockout) and anandamide uptake inhibitors, like AM404 and UCM707, affect both phases of formalin-induced pain in a way antagonized by CB1 receptor antagonists (Lichtman et al., 2004a, 2004b; La Rana et al., 2006; Sit et al., 2007). The possible reason for this discrepancy might be that these compounds were tested in previous studies at doses often higher than 5 mg kg−1, whereas in this study, only one compound (OMDM132) was tested at such high doses. Other possible explanations might be the different species (rat) used in some of the aforementioned studies with FAAH inhibitors, or the different amounts of formalin injected in the paw. Indeed, in a previous study carried out in rats with 5% formalin (Moore et al. 2005), LY2183240 affected the first phase of the formalin response when administered at a dose of 30 mg kg−1, whereas it was active on the second phase at a much lower dose, 10 mg kg−1.
In conclusion, we have provided here new evidence that some tetrazole-based inhibitors of endocannabinoid inactivation, such as LY2183240 and OMDM132, can inhibit pain through indirect activation of cannabinoid receptors, at least in part. We have also shown that both the presence and the absence of a highly reactive carbamoyl moiety in these tetrazole-based compounds is associated with non-cannabinoid receptor-mediated analgesia. Finally, we have found that selective inhibition of endocannabinoid uptake, with no effect on FAAH, as with OMDM132, can be accompanied by cannabinoid receptor-mediated antinociception, although with lower efficacy than that observed with dual FAAH/uptake inhibitors like LY2183240. Although limited to five compounds, our data point to an association between the capability of a tetrazole compound to inhibit selectively anandamide uptake or FAAH and its capability to exert an antinocifensive effect in the formalin test in a way dependent or not on cannabinoid receptors, respectively, thus confirming that the inhibition of anandamide uptake is pharmacologically distinguishable from FAAH blockade. However, direct data aiming at identifying the proteins responsible for the transport of endocannabinoids across the cell membrane still need to be obtained to substantiate the hypothesis that such process occurs also independently of FAAH.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- DAGL
diacylglycerol lipase
- FAAH
fatty acid amide hydrolase
Conflict of interest
The authors declare that they have no conflict of interest pertaining to the present data.
References
- Abbott FV, Franklin KB, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. doi: 10.1016/0304-3959(94)00095-V. [DOI] [PubMed] [Google Scholar]
- Alexander JP, Cravatt BF. The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J Am Chem Soc. 2006;128:9699–9704. doi: 10.1021/ja062999h. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors channels (GRAC) 3rd edition 2008 revision) Br J Pharmacol. 2008;153 Suppl. 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsani E, Labanca M, Bianchi R, Rodella LF. AM404 decreases Fos-immunoreactivity in the spinal cord in a model of inflammatory pain. Brain Res. 2007;1152:87–94. doi: 10.1016/j.brainres.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, et al. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli F, Giagnoni G, Bettoni I, Colleoni M, Costa B. The inhibition of monoacylglycerol lipase by URB602 showed an anti-inflammatory and anti-nociceptive effect in a murine model of acute inflammation. Br J Pharmacol. 2007;152:787–794. doi: 10.1038/sj.bjp.0707425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chem Phys Lipids. 2002;121:135–148. doi: 10.1016/s0009-3084(02)00147-0. [DOI] [PubMed] [Google Scholar]
- Dickason-Chesterfield AK, Kidd SR, Moore SA, Schaus JM, Liu B, Nomikos GG, et al. Pharmacological characterization of endocannabinoid transport and fatty acid amide hydrolase inhibitors. Cell Mol Neurobiol. 2006;26:407–423. doi: 10.1007/s10571-006-9072-6. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce. Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. The cannabinoid system and its pharmacological manipulation–a review, with emphasis upon the uptake and hydrolysis of anandamide. Fundam Clin Pharmacol. 2006;20:549–562. doi: 10.1111/j.1472-8206.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Giblin GM, O'Shaughnessy CT, Naylor A, Mitchell WL, Eatherton AJ, Slingsby BP. Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro- 2H-pyran-4-yl)methyl]-4-(trifluoromethyl)- 5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem. 2007;50:2597–2600. doi: 10.1021/jm061195+. [DOI] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol. 2007;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. A physiological role for endocannabinoid-derived products of cyclooxygenase-2-mediated oxidative metabolism. Br J Pharmacol. 2008;153:1341–1343. doi: 10.1038/bjp.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG. Inhibitors of monoacylglycerol lipase as novel analgesics. Br J Pharmacol. 2007;150:673–675. doi: 10.1038/sj.bjp.0707153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin. 2007;23:17–24. doi: 10.1185/030079906x158066. [DOI] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Sagar DR, Elmes SJ, Kendall DA, Chapman V. Cannabinoid CB2 receptor-mediated anti-nociception in models of acute and chronic pain. Mol Neurobiol. 2007;36:26–35. doi: 10.1007/s12035-007-8007-7. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Ohashi K, Sugie Y, Sugimoto H, Omura H. Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF-03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J Pharmacol Sci. 2008;106:219–224. doi: 10.1254/jphs.fp0071599. [DOI] [PubMed] [Google Scholar]
- La Rana G, Russo R, Campolongo P, Bortolato M, Mangieri RA, Cuomo V, et al. Modulation of neuropathic and inflammatory pain by the endocannabinoid transport inhibitor AM404 [N-(4-hydroxyphenyl)-eicosa-5,8,11,14-tetraenamide] J Pharmacol Exp Ther. 2006;317:1365–1371. doi: 10.1124/jpet.105.100792. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, et al. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004a;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004b;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Maida V, Ennis M, Irani S, Corbo M, Dolzhykov M. Adjunctive nabilone in cancer pain and symptom management: a prospective observational study using propensity scoring. J Support Oncol. 2008;6:119–124. [PubMed] [Google Scholar]
- Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Maione S, De Petrocellis L, de Novellis V, Moriello AS, Petrosino S, Palazzo E, et al. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br J Pharmacol. 2007;150:766–781. doi: 10.1038/sj.bjp.0707145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell VA, Greenwood R, Jayamanne A, Vaughan CW. Actions of the endocannabinoid transport inhibitor AM404 in neuropathic and inflammatory pain models. Clin Exp Pharmacol Physiol. 2007;34:1186–1190. doi: 10.1111/j.1440-1681.2007.04692.x. [DOI] [PubMed] [Google Scholar]
- Moore SA, Nomikos GG, Dickason-Chesterfield AK, Schober DA, Schaus JM, Ying BP, et al. Identification of a high-affinity binding site involved in the transport of endocannabinoids. Proc Natl Acad Sci USA. 2005;102:17852–17857. doi: 10.1073/pnas.0507470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. 2008;9:254–264. doi: 10.1016/j.jpain.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Ortar G, Cascio MG, Moriello AS, Camalli M, Morera E, Nalli M, et al. Carbamoyl tetrazoles as inhibitors of endocannabinoid inactivation: a critical revisitation. Eur J Med Chem. 2008a;43:62–72. doi: 10.1016/j.ejmech.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Ortar G, Schiano Moriello A, Cascio MG, De Petrocellis L, Ligresti A, Morera E, et al. New tetrazole-based selective anandamide uptake inhibitors. Bioorg Med Chem Lett. 2008b;18:2820–2824. doi: 10.1016/j.bmcl.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007;29:2068–2079. doi: 10.1016/j.clinthera.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- Saddi G, Abbott FV. The formalin test in the mouse: a parametric analysis of scoring properties. Pain. 2000;89:53–63. doi: 10.1016/S0304-3959(00)00348-1. [DOI] [PubMed] [Google Scholar]
- Sit SY, Conway C, Bertekap R, Xie K, Bourin C, Burris K, et al. Novel inhibitors of fatty acid amide hydrolase. Bioorg Med Chem Lett. 2007;17:3287–3291. doi: 10.1016/j.bmcl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–137. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br J Pharmacol. 2008;153:390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]