Abstract
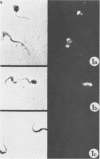
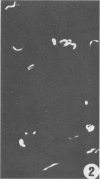
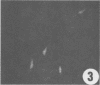
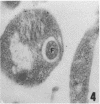
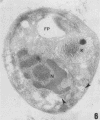
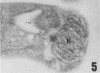
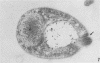
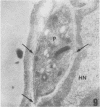
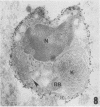
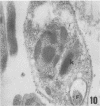
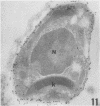
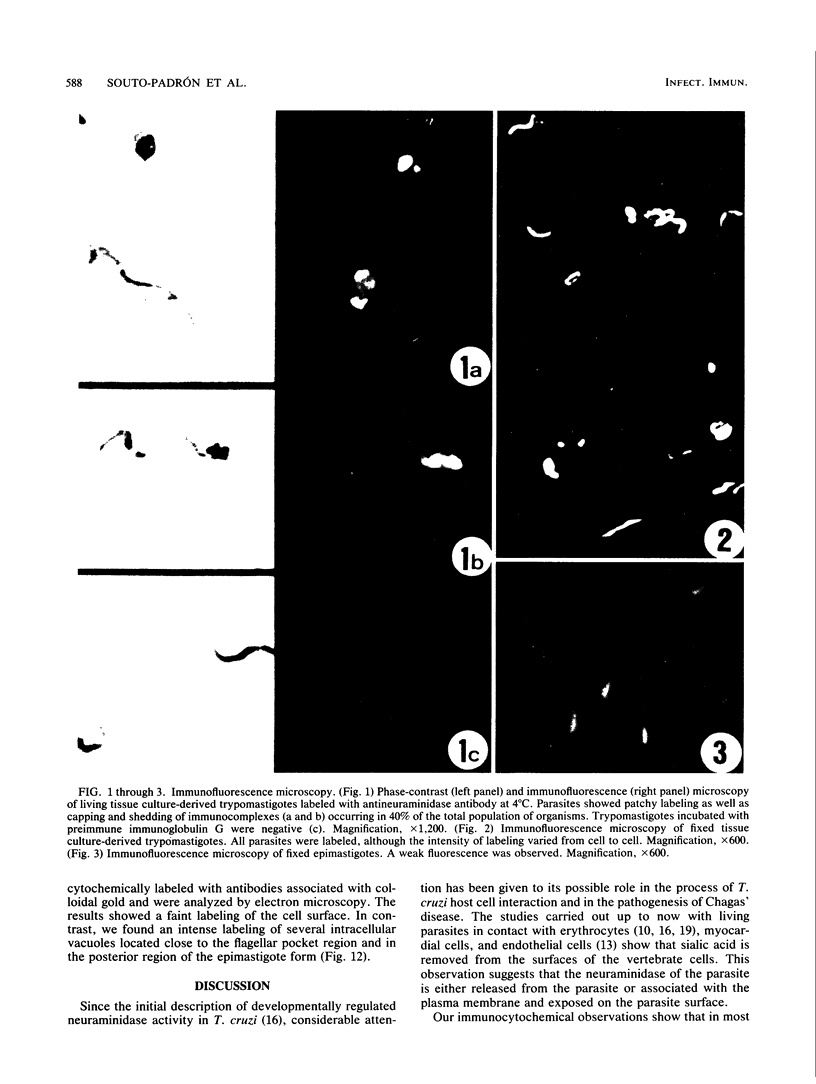
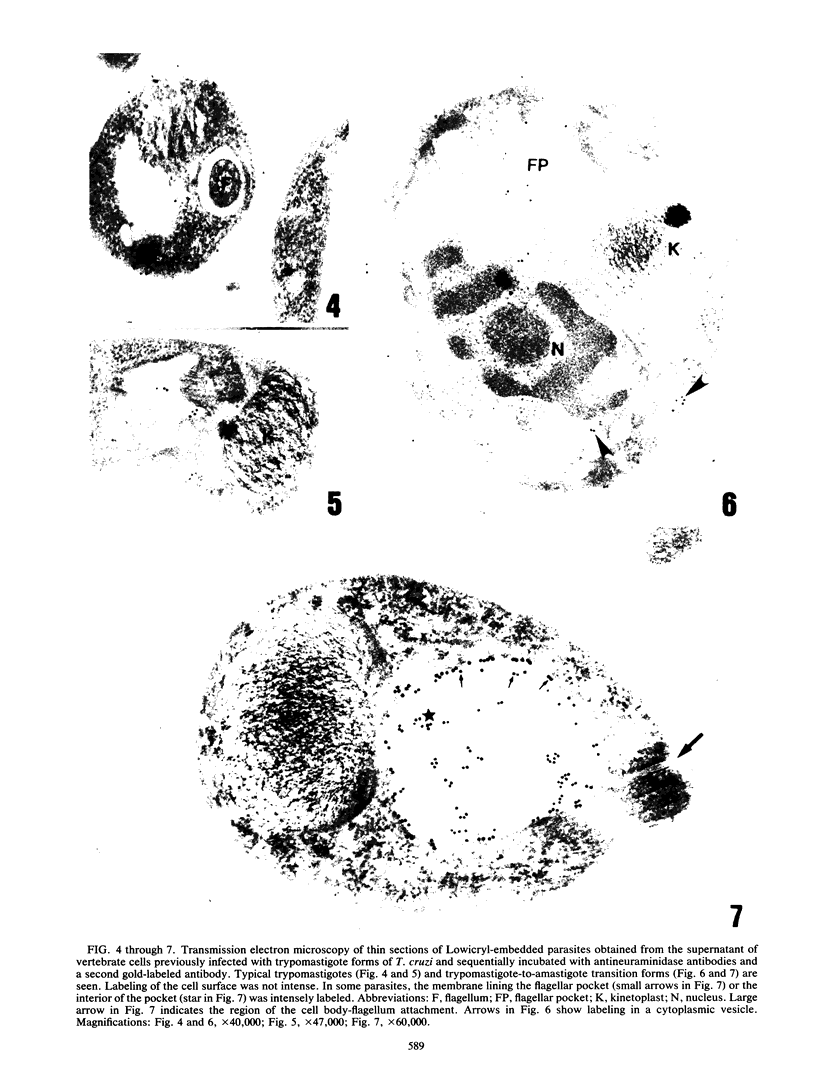
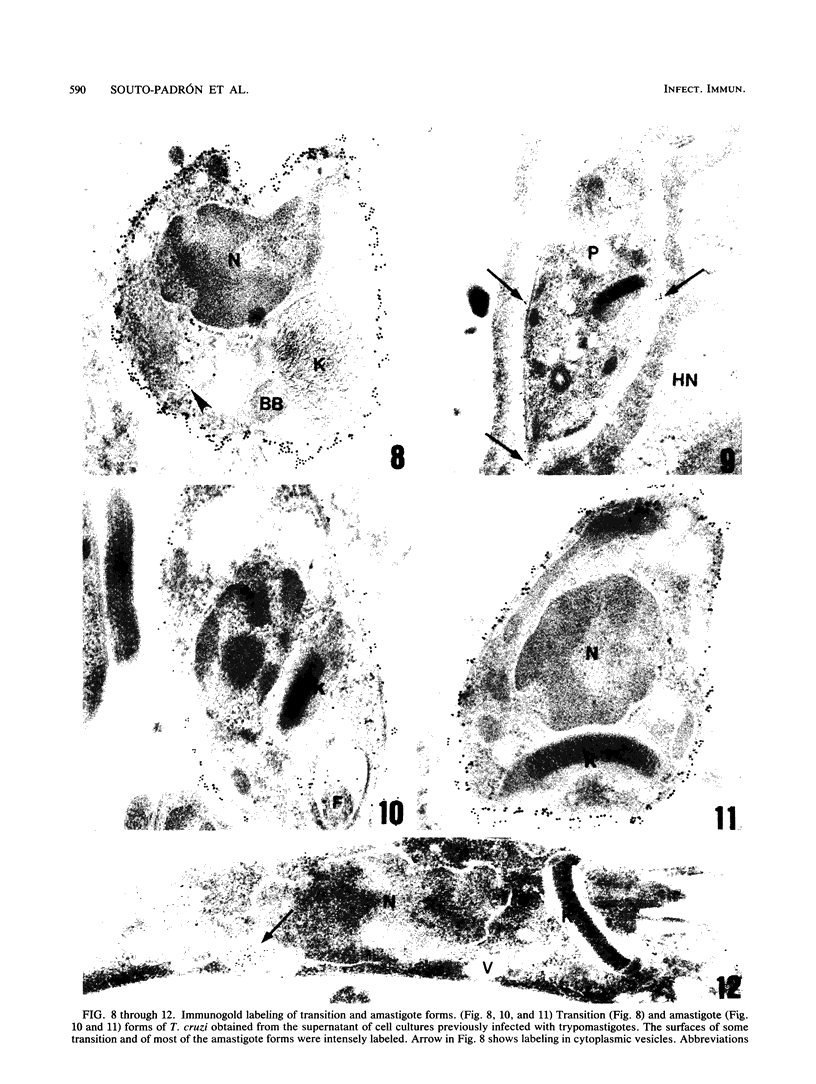
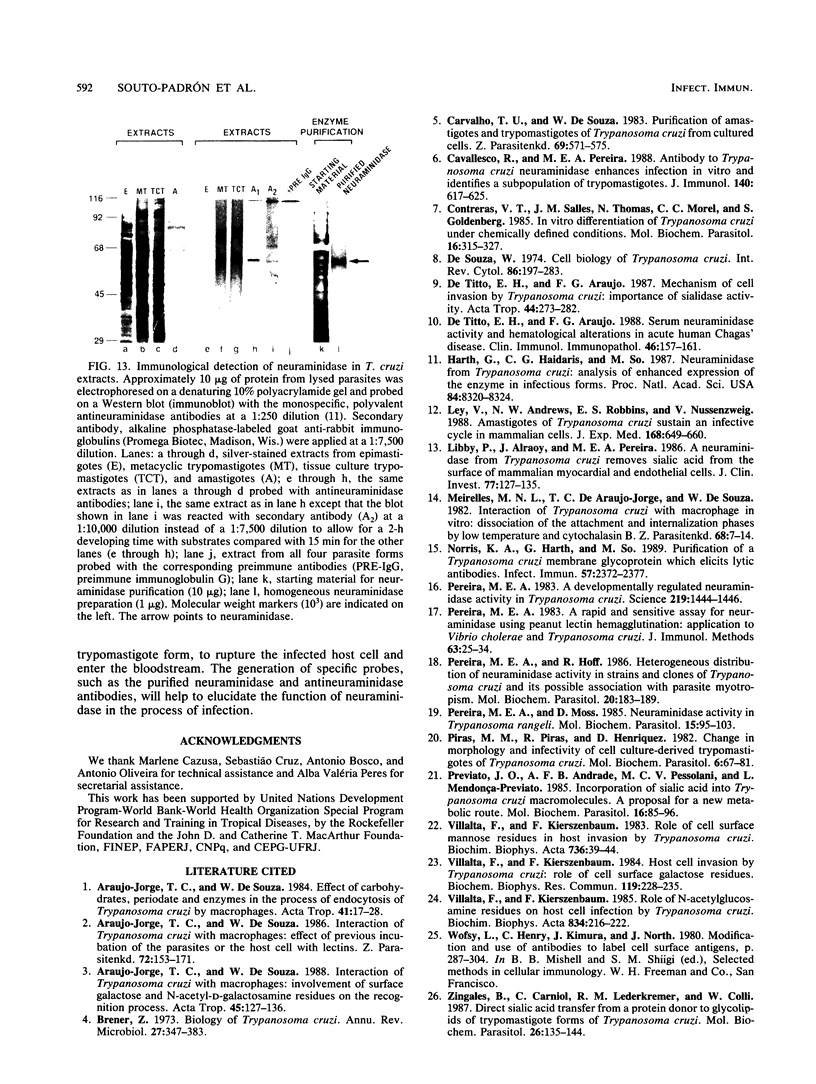
A polyclonal antibody obtained against neuraminidase purified from Trypanosoma cruzi was used for the localization of the protein in whole cells by immunofluorescence microscopy and in thin sections of parasites (epimastigote, amastigote, and trypomastigote forms) embedded at a low temperature in Lowicryl K4M resin. The intensity of labeling, as evaluated by the number of gold particles associated with the parasite, varied according to the protozoan developmental stage. In the noninfective epimastigote forms, labeling of the cell surface was very weak. However, an intense labeling of some cytoplasmic vacuoles was observed. Labeling of the surfaces of most of the trypomastigote forms was weak, while gold particles were seen in association with the flagellar pockets of these forms, which suggests that the enzyme is secreted through this region. Intense labeling of the surfaces of many, but not all, transition forms between trypomastigote and amastigote forms was observed. Amastigote forms found in the supernatant of infected cell cultures had their surfaces intensely labeled, while few particles were seen on the surfaces of intracellular amastigotes. The results obtained are discussed in relation to the role played by T. cruzi neuraminidase in the process of parasite-host cell interaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araújo-Jorge T. C., De Souza W. Interaction of Trypanosoma cruzi with macrophages. Involvement of surface galactose and N-acetyl-D-galactosamine residues on the recognition process. Acta Trop. 1988 Jun;45(2):127–136. [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Cavallesco R., Pereira M. E. Antibody to Trypanosoma cruzi neuraminidase enhances infection in vitro and identifies a subpopulation of trypomastigotes. J Immunol. 1988 Jan 15;140(2):617–625. [PubMed] [Google Scholar]
- Contreras V. T., Salles J. M., Thomas N., Morel C. M., Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985 Sep;16(3):315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- Harth G., Haidaris C. G., So M. Neuraminidase from Trypanosoma cruzi: analysis of enhanced expression of the enzyme in infectious forms. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8320–8324. doi: 10.1073/pnas.84.23.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley V., Andrews N. W., Robbins E. S., Nussenzweig V. Amastigotes of Trypanosoma cruzi sustain an infective cycle in mammalian cells. J Exp Med. 1988 Aug 1;168(2):649–659. doi: 10.1084/jem.168.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Alroy J., Pereira M. E. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986 Jan;77(1):127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. A., Harth G., So M. Purification of a Trypanosoma cruzi membrane glycoprotein which elicits lytic antibodies. Infect Immun. 1989 Aug;57(8):2372–2377. doi: 10.1128/iai.57.8.2372-2377.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pereira M. E. A rapid and sensitive assay for neuraminidase using peanut lectin hemagglutination: application to Vibrio cholera and Trypanosoma cruzi. J Immunol Methods. 1983 Sep 30;63(1):25–34. doi: 10.1016/0022-1759(83)90206-5. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Hoff R. Heterogeneous distribution of neuraminidase activity in strains and clones of Trypanosoma cruzi and its possible association with parasite myotropism. Mol Biochem Parasitol. 1986 Aug;20(2):183–189. doi: 10.1016/0166-6851(86)90030-7. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Moss D. Neuraminidase activity in Trypanosoma rangeli. Mol Biochem Parasitol. 1985 Apr;15(1):95–103. doi: 10.1016/0166-6851(85)90031-3. [DOI] [PubMed] [Google Scholar]
- Piras M. M., Piras R., Henriquez D., Negri S. Changes in morphology and infectivity of cell culture-derived trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 1982 Aug;6(2):67–81. doi: 10.1016/0166-6851(82)90066-4. [DOI] [PubMed] [Google Scholar]
- Previato J. O., Andrade A. F., Pessolani M. C., Mendonça-Previato L. Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol Biochem Parasitol. 1985 Jun;16(1):85–96. doi: 10.1016/0166-6851(85)90051-9. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Host cell invasion by Trypanosoma cruzi: role of cell surface galactose residues. Biochem Biophys Res Commun. 1984 Feb 29;119(1):228–235. doi: 10.1016/0006-291x(84)91642-5. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of cell surface mannose residues in host cell invasion by Trypanosoma cruzi. Biochim Biophys Acta. 1983 Dec 7;736(1):39–44. doi: 10.1016/0005-2736(83)90167-0. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of surface N-acetylglucosamine residues on host cell infection by Trypanosoma cruzi. Biochim Biophys Acta. 1985 May 30;845(2):216–222. doi: 10.1016/0167-4889(85)90179-x. [DOI] [PubMed] [Google Scholar]
- Zingales B., Carniol C., de Lederkremer R. M., Colli W. Direct sialic acid transfer from a protein donor to glycolipids of trypomastigote forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1987 Nov;26(1-2):135–144. doi: 10.1016/0166-6851(87)90137-x. [DOI] [PubMed] [Google Scholar]
- de Araújo Jorge T. C., de Souza W. Effect of carbohydrates, periodate and enzymes in the process of endocytosis of Trypanosoma cruzi by macrophages. Acta Trop. 1984 Mar;41(1):17–28. [PubMed] [Google Scholar]
- de Araújo-Jorge T. C., de Souza W. Interaction of Trypanosoma cruzi with macrophages: effect of previous incubation of the parasites or the host cells with lectins. Z Parasitenkd. 1986;72(2):153–171. doi: 10.1007/BF00931143. [DOI] [PubMed] [Google Scholar]
- de Carvalho T. U., de Souza W. Separation of amastigotes and trypomastigotes of Trypanosoma cruzi from cultured cells. Z Parasitenkd. 1983;69(5):571–575. doi: 10.1007/BF00926668. [DOI] [PubMed] [Google Scholar]
- de Meirelles M. N., de Araújo Jorge T. C., de Souza W. Interaction of Trypanosoma cruzi with macrophages in vitro: dissociation of the attachment and internalization phases by low temperature and cytochalasin B. Z Parasitenkd. 1982;68(1):7–14. doi: 10.1007/BF00926652. [DOI] [PubMed] [Google Scholar]
- de Souza W. Cell biology of Trypanosoma cruzi. Int Rev Cytol. 1984;86:197–283. doi: 10.1016/s0074-7696(08)60180-1. [DOI] [PubMed] [Google Scholar]
- de Titto E. H., Araujo F. G. Mechanism of cell invasion by Trypanosoma cruzi: importance of sialidase activity. Acta Trop. 1987 Sep;44(3):273–282. [PubMed] [Google Scholar]
- de Titto E. H., Araujo F. G. Serum neuraminidase activity and hematological alterations in acute human Chagas' disease. Clin Immunol Immunopathol. 1988 Jan;46(1):157–161. doi: 10.1016/0090-1229(88)90016-5. [DOI] [PubMed] [Google Scholar]