Abstract
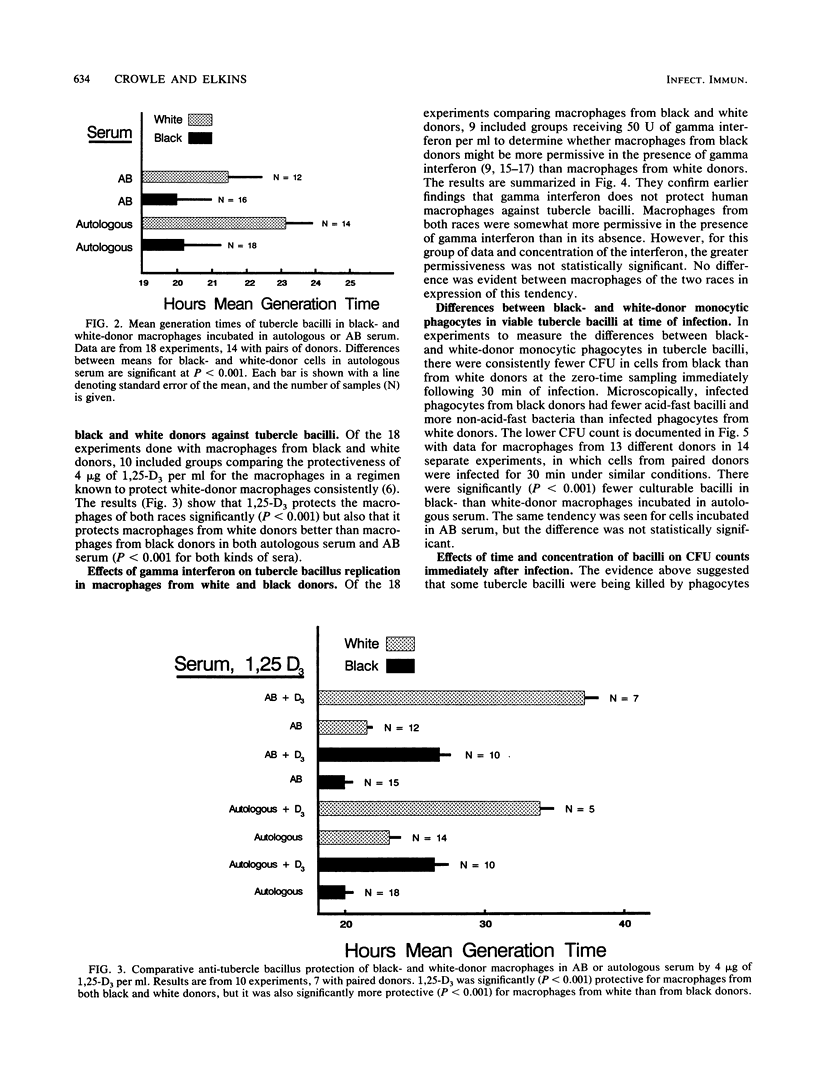
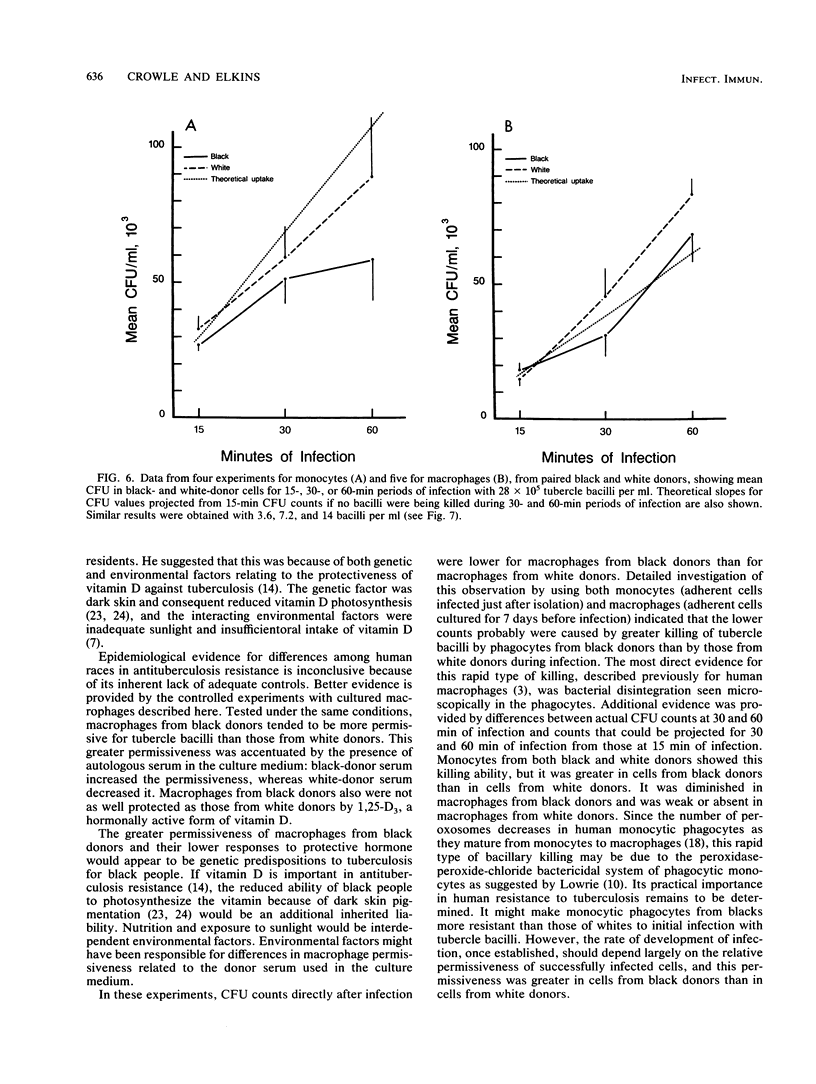
Epidemiological, clinical, and histopathological evidence suggests that black people are more susceptible to tuberculosis than are white people. The cellular basis of this putative susceptibility was investigated in vitro by comparing responses of blood-derived macrophages from black and white donors to experimental infection with virulent tubercle bacilli. Phagocytes from pairs of black and white donors were infected. The uptake and replication of the tubercle bacilli in these cells were measured by microscopic counts and by CFU counts of bacilli at 0, 4, and 7 days. The effects of donor serum, of 1,25-(OH)2-vitamin D3, and gamma interferon on the infection also were studied. Black-donor phagocytes killed more bacilli during phagocytosis than white-donor phagocytes did. However, the bacilli grew consistently and significantly faster in successfully infected macrophages from black than from white donors, especially in the presence of black-donor serum. 1,25-(OH)2-vitamin D3 gave significantly less protection against tubercle bacilli to macrophages from black donors than to macrophages from white donors. The permissiveness of the macrophages from the two races was affected equally by gamma interferon. These results demonstrate some inherent and environmental liabilities in the monocytic phagocytes and serum of black people compared with white people, which may contribute to their greater susceptibility to tuberculosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowle A. J., May M. H. Replication of lyophilized and cultured BCG in human macrophages. Am Rev Respir Dis. 1983 Oct;128(4):673–679. doi: 10.1164/arrd.1983.128.4.673. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., May M. Preliminary demonstration of human tuberculoimmunity in vitro. Infect Immun. 1981 Jan;31(1):453–464. doi: 10.1128/iai.31.1.453-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Ross E. J., May M. H. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987 Dec;55(12):2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. D. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle. 1985 Dec;66(4):301–306. doi: 10.1016/0041-3879(85)90068-6. [DOI] [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray D. N., Bartow R. A., Mintzer C. L. Impact of protein malnutrition on exogenous reinfection with Mycobacterium tuberculosis. Infect Immun. 1989 Jun;57(6):1746–1749. doi: 10.1128/iai.57.6.1746-1749.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986 Jan;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. The role of vitamin D in tuberculosis. Am Rev Respir Dis. 1988 Oct;138(4):768–770. doi: 10.1164/ajrccm/138.4.768. [DOI] [PubMed] [Google Scholar]
- Sanderson R. J., Shepperdson R. T., Vatter A. E., Talmage D. W. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977 Apr;118(4):1409–1414. [PubMed] [Google Scholar]
- Snider D. E., Jr, Hutton M. D. Tuberculosis in correctional institutions. JAMA. 1989 Jan 20;261(3):436–437. [PubMed] [Google Scholar]
- Snider D. E. Reorientation of tuberculosis control programmes in the USA. Bull Int Union Tuberc Lung Dis. 1989 Mar;64(1):25–26. [PubMed] [Google Scholar]
- Webb A. R., Holick M. F. The role of sunlight in the cutaneous production of vitamin D3. Annu Rev Nutr. 1988;8:375–399. doi: 10.1146/annurev.nu.08.070188.002111. [DOI] [PubMed] [Google Scholar]
- Webb A. R., Kline L., Holick M. F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988 Aug;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]


