Abstract
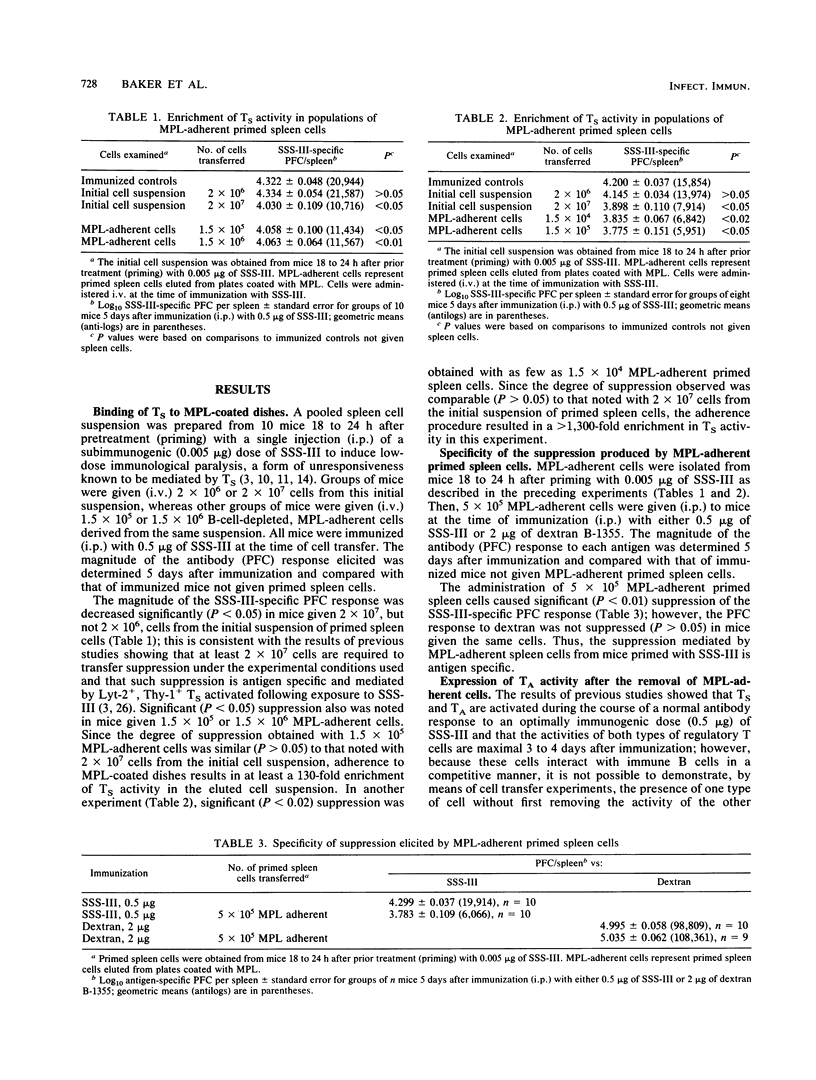
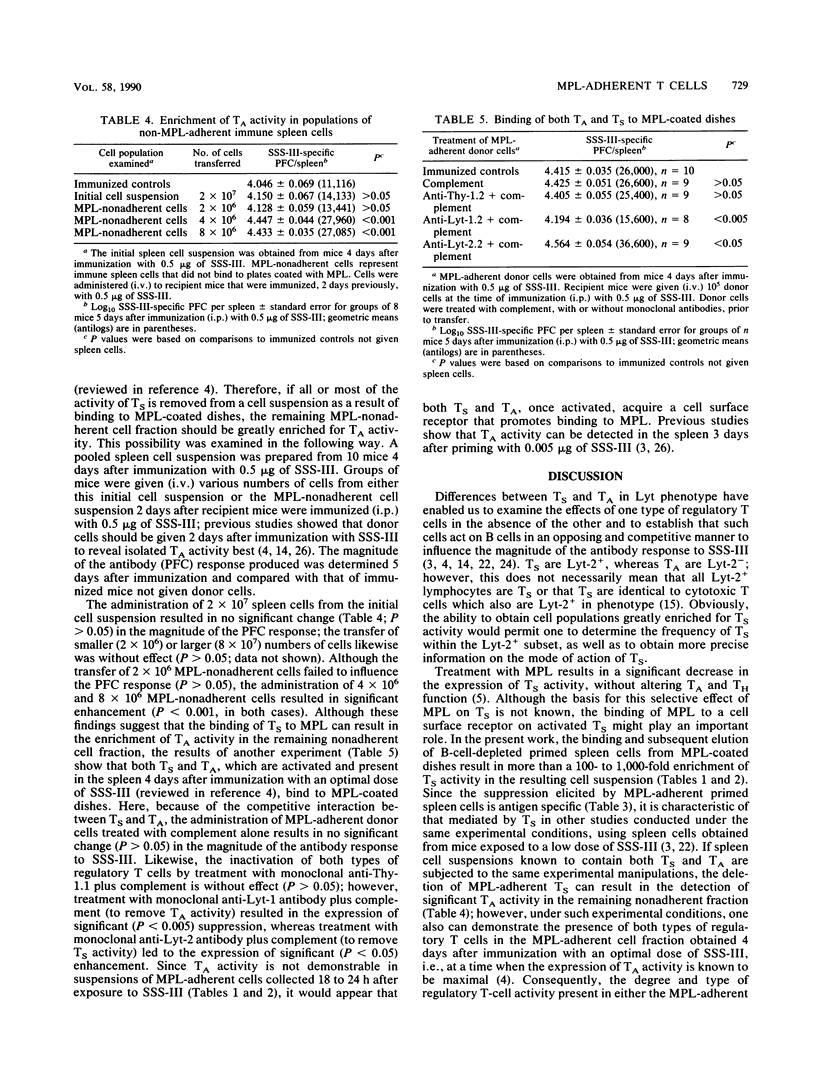
The binding and elution of spleen cells from plastic dishes coated with monophosphoryl lipid A (MPL) resulted in a greater than 1,000-fold enrichment of antigen-specific suppressor T-cell (TS) activity when spleen cells from mice 18 to 24 h after exposure to a low dose of type III pneumonococcal polysaccharide (SSS-III) were used. The removal of MPL-adherent TS cells resulted in an increase in the degree of amplifier T-cell (TA) activity present in the remaining MPL-nonadherent cell fraction; however, both TS and TA activities were found in the MPL-adherent cell fraction when spleen cells from mice 4 days after immunization with an optimal dose of SSS-III were examined. These findings, as well as others, suggest that both TS and TA, once activated, acquire a cell surface receptor that enables them to bind to MPL. Because of differences in the kinetics for the activation of TS and TA during the course of the antibody response and the fact that TS, but not TA, activity appears as early as 18 to 24 h after exposure to SSS-III, it is possible to use this experimental approach to obtain cell suspensions greatly enriched in TS activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Direct evidence for the involvement of T suppressor cells in the expression of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1982 Mar;128(3):1059–1062. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Regulation of the antibody response to pneumococcal polysaccharide by thymus-derived cells. Rev Infect Dis. 1981 Mar-Apr;3(2):332–341. doi: 10.1093/clinids/3.2.332. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Fauntleroy M. B., Prescott B. Examination of the differential characteristics of amplifier and contrasuppressor T cells. Immunobiology. 1988 Sep;177(4-5):438–448. doi: 10.1016/S0171-2985(88)80010-X. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J. Homeostatic control of antibody responses: a model based on the recognition of cell-associated antibody by regulatory T cells. Transplant Rev. 1975;26:3–20. doi: 10.1111/j.1600-065x.1975.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Prescott B., Stashak P. W., Amsbaugh D. F. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. 3. Studies on the average avidity of the antibody produced by specific plaque-forming cells. J Immunol. 1971 Sep;107(3):719–724. [PubMed] [Google Scholar]
- Baker P. J., Rudbach J. A., Prescott B., Caldes G., Evans C., Stashak P. W. Influence of multiple genes on the magnitude of the antibody response to bacterial polysaccharide antigens. Infect Immun. 1984 Jul;45(1):56–61. doi: 10.1128/iai.45.1.56-61.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Taylor C., Fauntleroy M. B., Stashak P. W., Prescott B. The role of antigen in the activation of regulatory T cells by immune B cells. Cell Immunol. 1985 Dec;96(2):376–385. doi: 10.1016/0008-8749(85)90368-5. [DOI] [PubMed] [Google Scholar]
- Fan J., Ahmed A., Bonavida B. Studies on the induction and expression of T cell-mediated immunity. X. Inhibition by Lyt 2,3 antisera of cytotoxic T lymphocyte-mediated antigen-specific and -nonspecific cytotoxicity: evidence for the blocking of the binding between T lymphocytes and target cells and not the post-binding cytolytic steps. J Immunol. 1980 Dec;125(6):2444–2453. [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Hiernaux J. R., Stashak P. W., Cantrell J. L., Rudbach J. A., Baker P. J. Immunomodulatory activity of monophosphoryl lipid A in C3H/HeJ and C3H/HeSnJ mice. Infect Immun. 1989 May;57(5):1483–1490. doi: 10.1128/iai.57.5.1483-1490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Prescott B. Kinetics of the antibody response to type III pneumococcal polysaccharide (SSS-III). I. Use of 125I-labeled SSS-III to study serum antibody levels, as well as the distribution and excretion of antigen after immunization. J Immunol. 1976 Jan;116(1):41–51. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. II. Membrane localization and binding characteristics. J Immunol. 1988 Aug 1;141(3):1006–1011. [PubMed] [Google Scholar]
- McCoy K. L., Baker P. J., Stashak P. W., Chused T. M. Two defects in old New Zealand Black mice are involved in the loss of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1985 Oct;135(4):2438–2442. [PubMed] [Google Scholar]
- Ribi E. Beneficial modification of the endotoxin molecule. J Biol Response Mod. 1984;3(1):1–9. [PubMed] [Google Scholar]
- Taylor C. E., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B., Baker P. J. Cell surface antigens and other characteristics of T cells regulating the antibody response to type III pneumococcal polysaccharide. J Immunol. 1983 Jan;130(1):19–23. [PubMed] [Google Scholar]
- Taylor C. E., Bright R. T-cell modulation of the antibody response to bacterial polysaccharide antigens. Infect Immun. 1989 Jan;57(1):180–185. doi: 10.1128/iai.57.1.180-185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. E., Stashak P. W., Caldes G., Prescott B., Chused T. E., Brooks A., Baker P. J. Activation of antigen-specific suppressor T cells by B cells from mice immunized with type III pneumococcal polysaccharide. J Exp Med. 1983 Sep 1;158(3):703–717. doi: 10.1084/jem.158.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]


