Abstract
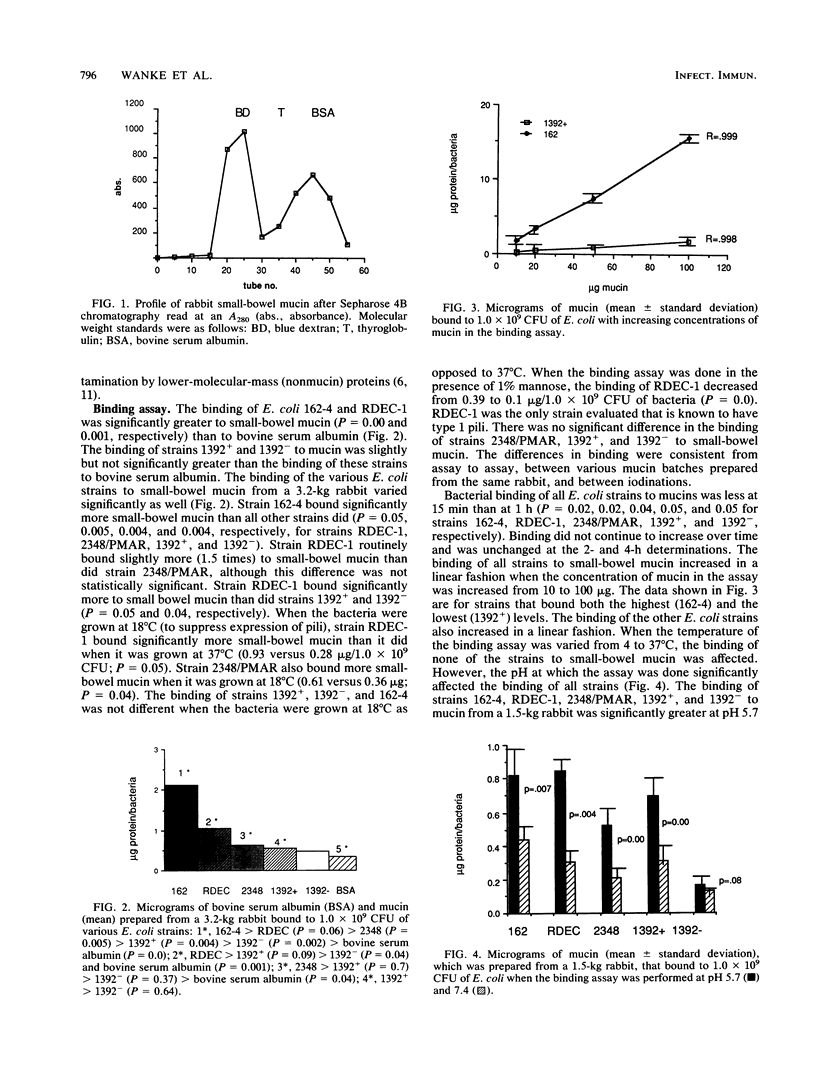
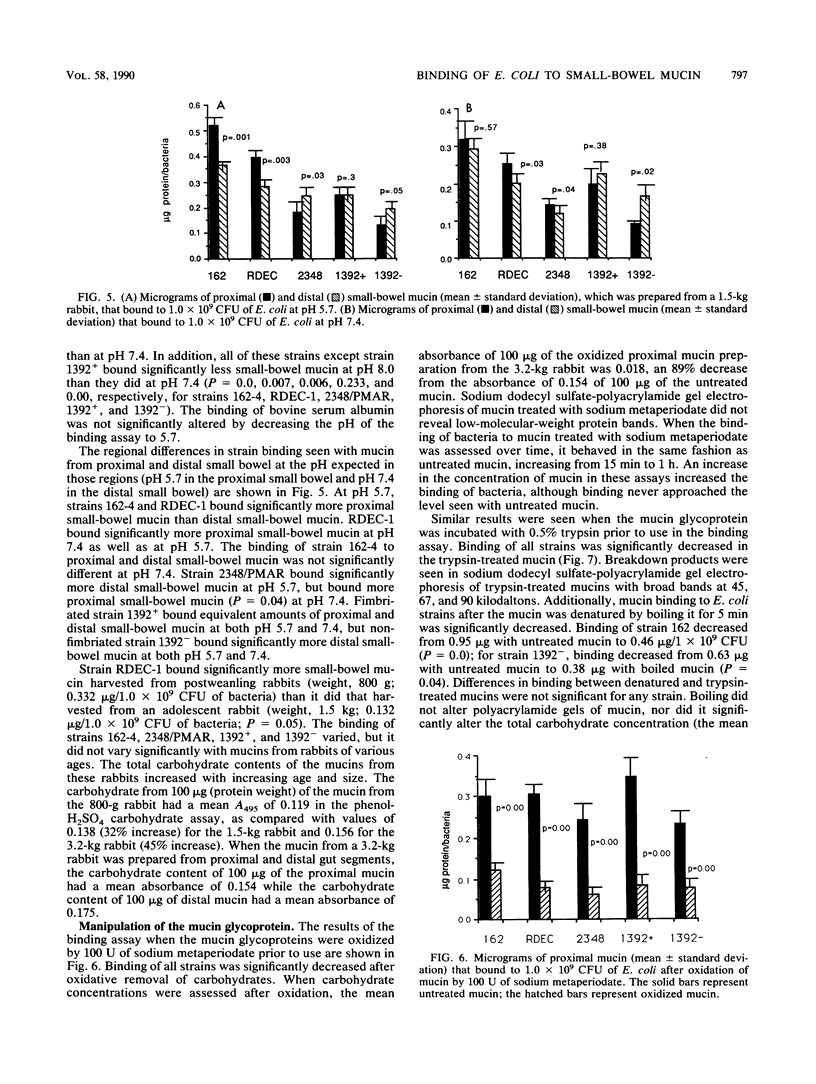
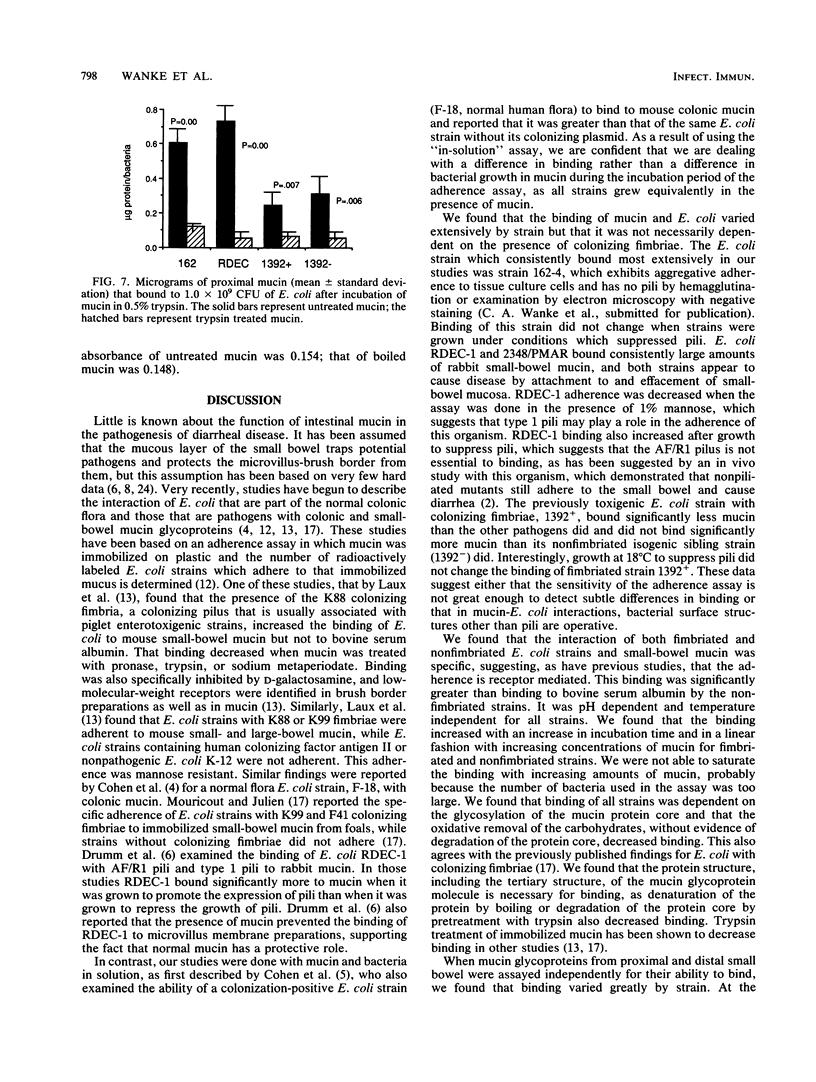
Before an enteropathogen binds to the small bowel, it must interact with the small-bowel mucus (SBM) layer. To determine whether this interaction involves specific binding of diarrheagenic Escherichia coli, we used a quantitative assay with labeled, purified rabbit SBM. Binding of SBM from an adult rabbit was significantly greater to strain 162, an agglutinating E. coli strain, than it was to RDEC-1, a rabbit pathogen, and was significantly greater to strain 2348/PMAR, an enteropathogenic E. coli strain, than it was to strains 1392+ and 1392-, which are enterotoxigenic E. coli strains with and without colonizing fimbriae, respectively. Binding of strains RDEC-1, 2348/PMAR, and 162-4 was significantly greater to SBM than to bovine serum albumin. Binding of all strains increased in a linear fashion with increasing amounts of SBM and was reproducible (r = 0.85). Binding was significantly greater at pH 5.7 than at pH 7.4 or 8.0 for all five strains. Temperature did not alter the binding of any strain. Strains 162-4 and RDEC-1 bound significantly more to proximal SBM than to rabbit distal SBM, while strains 1392+ and 1392- bound significantly more to distal SBM. Oxidation of sugars from SBM significantly decreased the binding of all strains. Each pathogenic E. coli strain bound distinctively to SBM; the SBM sugars appeared to mediate this binding for all E. coli strains. Binding was also dependent on mucin characteristics, as binding varied by region of the gut (increased for proximal SBM for strains 162-4 and RDEC-1 and for distal SBM for strains 1392+ and 1392-). The developmental age of the gut significantly affected binding only of the rabbit pathogen RDEC-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Inman L. R., Blake R. K. Production of diarrhea in the rabbit by a mutant of Escherichia coli (RDEC-1) that does not express adherence (AF/R1) pili. J Infect Dis. 1989 Jul;160(1):136–141. doi: 10.1093/infdis/160.1.136. [DOI] [PubMed] [Google Scholar]
- Chadee K., Petri W. A., Jr, Innes D. J., Ravdin J. I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm B., Roberton A. M., Sherman P. M. Inhibition of attachment of Escherichia coli RDEC-1 to intestinal microvillus membranes by rabbit ileal mucus and mucin in vitro. Infect Immun. 1988 Sep;56(9):2437–2442. doi: 10.1128/iai.56.9.2437-2442.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F. Intestinal mucins in health and disease. Digestion. 1978;17(3):234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- Forstner J., Wesley A., Mantle M., Kopelman H., Man D., Forstner G. Abnormal mucus: nominated but not yet elected. J Pediatr Gastroenterol Nutr. 1984;3 (Suppl 1):S67–S73. [PubMed] [Google Scholar]
- Gilman R. H., Partanen R., Brown K. H., Spira W. M., Khanam S., Greenberg B., Bloom S. R., Ali A. Decreased gastric acid secretion and bacterial colonization of the stomach in severely malnourished Bangladeshi children. Gastroenterology. 1988 Jun;94(6):1308–1314. doi: 10.1016/0016-5085(88)90668-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laux D. C., McSweegan E. F., Williams T. J., Wadolkowski E. A., Cohen P. S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect Immun. 1986 Apr;52(1):18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Rennels M. B., Daya V., Hughes T. P. Hemagglutination and colonization factors in enterotoxigenic and enteropathogenic Escherichia coli that cause diarrhea. J Infect Dis. 1980 Jun;141(6):733–737. doi: 10.1093/infdis/141.6.733. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Mouricout M. A., Julien R. A. Pilus-mediated binding of bovine enterotoxigenic Escherichia coli to calf small intestinal mucins. Infect Immun. 1987 May;55(5):1216–1223. doi: 10.1128/iai.55.5.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Kaper J. B., Robins-Browne R., Prado V., Vial P., Levine M. M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987 Sep;6(9):829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Maher K. O., Mackie P., Kaper J. B. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1987 Oct;55(10):2370–2377. doi: 10.1128/iai.55.10.2370-2377.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev Infect Dis. 1987 Jan-Feb;9(1):28–53. doi: 10.1093/clinids/9.1.28. [DOI] [PubMed] [Google Scholar]
- Satterwhite T. K., Evans D. G., DuPont H. L., Evans D. J., Jr Role of Escherichia coli colonisation factor antigen in acute diarrhoea. Lancet. 1978 Jul 22;2(8082):181–184. doi: 10.1016/s0140-6736(78)91921-9. [DOI] [PubMed] [Google Scholar]
- Sherman P. M., Boedeker E. C. Pilus-mediated interactions of the Escherichia coli strain RDEC-1 with mucosal glycoproteins in the small intestine of rabbits. Gastroenterology. 1987 Oct;93(4):734–743. doi: 10.1016/0016-5085(87)90435-5. [DOI] [PubMed] [Google Scholar]
- Sherman P. M., Boedeker E. C. Regional differences in attachment of enteroadherent Escherichia coli strain RDEC-1 to rabbit intestine: luminal colonization but lack of mucosal adherence in jejunal self-filling blind loops. J Pediatr Gastroenterol Nutr. 1987 May-Jun;6(3):439–444. doi: 10.1097/00005176-198705000-00022. [DOI] [PubMed] [Google Scholar]
- Sherman P., Fleming N., Forstner J., Roomi N., Forstner G. Bacteria and the mucus blanket in experimental small bowel bacterial overgrowth. Am J Pathol. 1987 Mar;126(3):527–534. [PMC free article] [PubMed] [Google Scholar]
- Snyder J. D., Walker W. A. Structure and function of intestinal mucin: developmental aspects. Int Arch Allergy Appl Immunol. 1987;82(3-4):351–356. doi: 10.1159/000234225. [DOI] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Vial P. A., Robins-Browne R., Lior H., Prado V., Kaper J. B., Nataro J. P., Maneval D., Elsayed A., Levine M. M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988 Jul;158(1):70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R., Guay C. M., DesJardins D., Pier G. B. Respiratory-mucin inhibition of the opsonophagocytic killing of Pseudomonas aeruginosa. Infect Immun. 1988 Sep;56(9):2218–2222. doi: 10.1128/iai.56.9.2218-2222.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke C. A., Guerrant R. L. Small-bowel colonization alone is a cause of diarrhea. Infect Immun. 1987 Aug;55(8):1924–1926. doi: 10.1128/iai.55.8.1924-1926.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


