Abstract
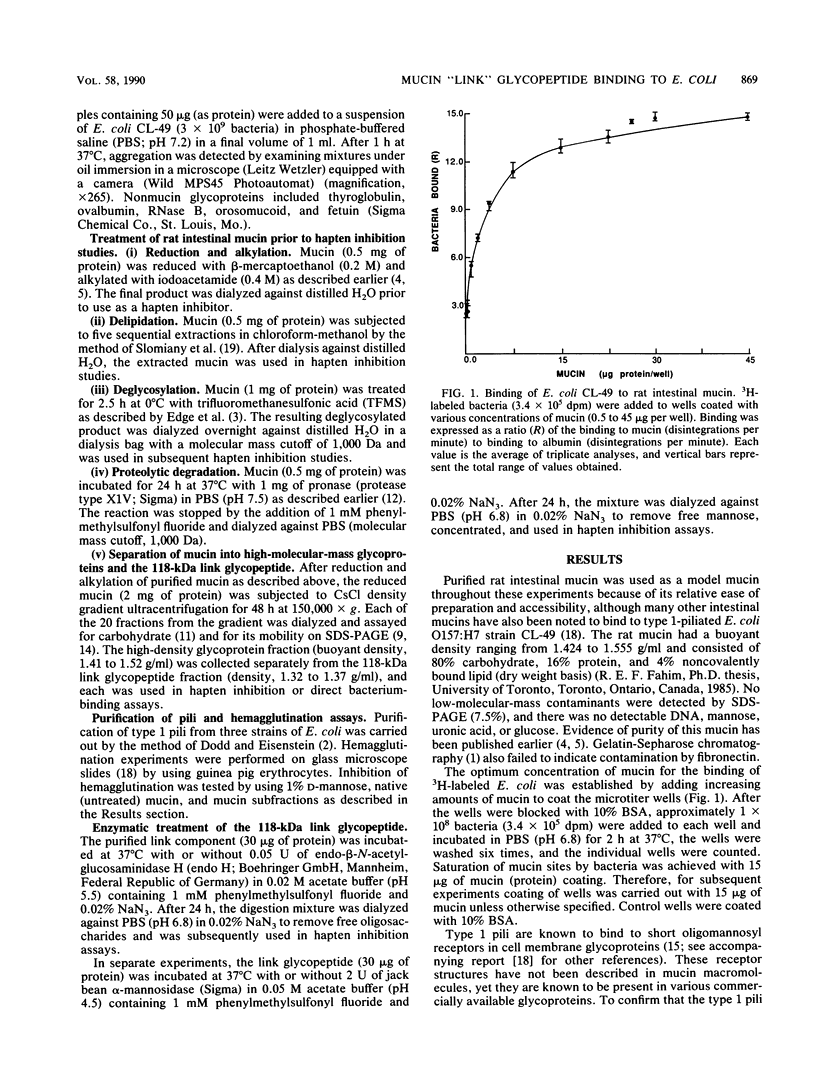
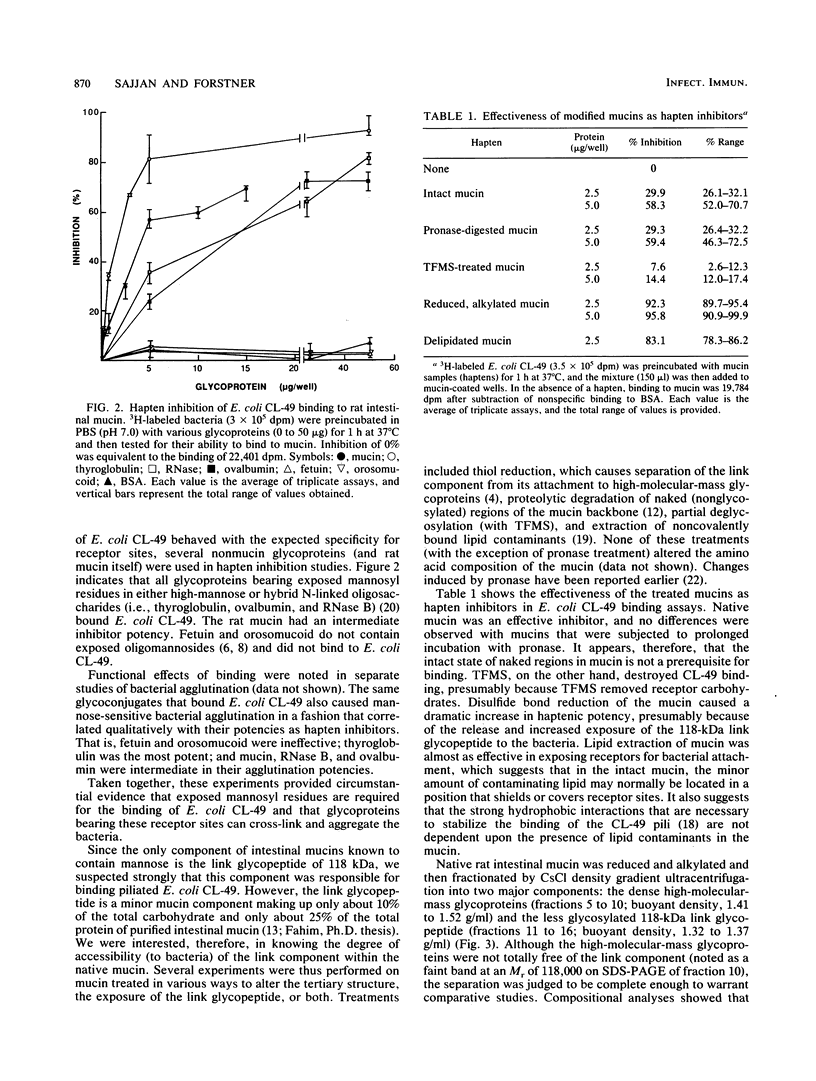
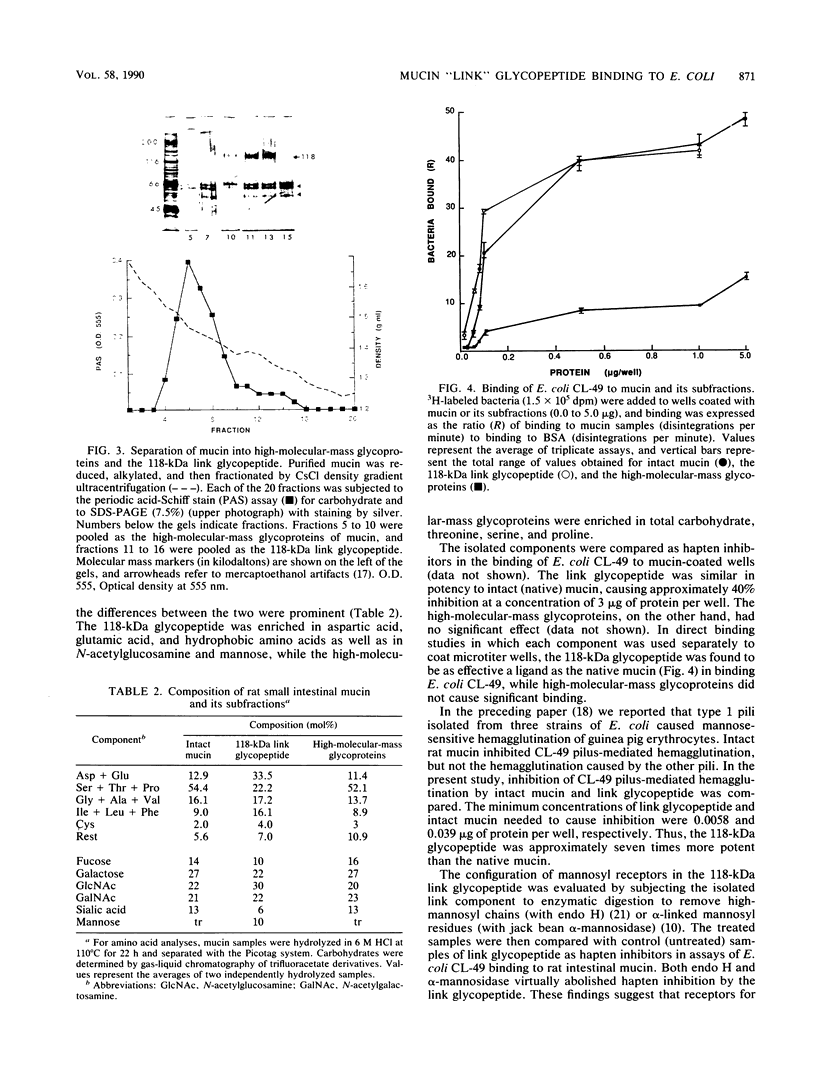
Purified rat intestinal mucin was used to identify mucin-binding sites for type 1-piliated Escherichia coli O157:H7 strain CL-49 isolated from a patient with hemorrhagic colitis and hemolytic uremic syndrome. Optimum binding of bacteria in a microtiter binding assay occurred with a mucin coating concentration of 15 micrograms (protein)/150 microliters. In hapten inhibition studies, several nonmucin glycoproteins bearing exposed mannosyl residues in N-linked oligosaccharides were effective inhibitors, as was rat mucin. The same glycoproteins caused bacterial aggregation. High-molecular-mass glycoproteins of the mucin were separated from its 118-kilodalton "link" glycopeptide fraction, and the latter was shown to be the mucin-binding component for E. coli CL-49 and its purified type 1 pili. This was confirmed in hemagglutination inhibition studies. Treatment of the link glycopeptide with jack bean alpha-mannosidase or endo-beta-N-acetylglucosaminidase H destroyed bacterial binding activity. Chemical or enzymatic modifications of intact rat mucin were undertaken to evaluate the normal accessibility of the link glycopeptide receptors to E. coli CL-49. Deglycosylation with trifluoromethane-sulfonic acid abolished binding, whereas pronase digestion had no effect. Reduction and alkylation as well as lipid extraction enhanced bacterial binding by the mucin, presumably by causing greater exposure of receptor sites. In summary, our binding studies revealed, for the first time, that intestinal mucin bears oligomannosyl receptors for type 1 pili and that these receptors are located on N-linked oligosaccharides of the 118-kilodalton link glycopeptide region of the mucin. Our experiments suggest the receptors are normally partly "covered" by noncovalently bound lipid. In addition, release of the link component from the rest of the mucin by disulfide bond reduction causes greater exposure of specific bacterium-binding sites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya S. N., Kaufman B., Khorrami A., Enriquez J. I., Manna B. Fibronectin: source of mannose in a highly purified respiratory mucin. Inflammation. 1988 Oct;12(5):433–446. doi: 10.1007/BF00919437. [DOI] [PubMed] [Google Scholar]
- Dodd D. C., Eisenstein B. I. Antigenic quantitation of type 1 fimbriae on the surface of Escherichia coli cells by an enzyme-linked immunosorbent inhibition assay. Infect Immun. 1982 Nov;38(2):764–773. doi: 10.1128/iai.38.2.764-773.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim R. E., Specian R. D., Forstner G. G., Forstner J. F. Characterization and localization of the putative 'link' component in rat small-intestinal mucin. Biochem J. 1987 May 1;243(3):631–640. doi: 10.1042/bj2430631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Y. T. Studies on the glycosidases in jack bean meal. I. Isolation and properties of alpha-mannosidase. J Biol Chem. 1967 Dec 10;242(23):5474–5480. [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Antigenic and structural features of goblet-cell mucin of human small intestine. Biochem J. 1984 Jan 1;217(1):159–167. doi: 10.1042/bj2170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Neeser J. R., Koellreutter B., Wuersch P. Oligomannoside-type glycopeptides inhibiting adhesion of Escherichia coli strains mediated by type 1 pili: preparation of potent inhibitors from plant glycoproteins. Infect Immun. 1986 May;52(2):428–436. doi: 10.1128/iai.52.2.428-436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Characteristics of binding of Escherichia coli serotype O157:H7 strain CL-49 to purified intestinal mucin. Infect Immun. 1990 Apr;58(4):860–867. doi: 10.1128/iai.58.4.860-867.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany A., Slomiany B. L., Witas H., Aono M., Newman L. J. Isolation of fatty acids covalently bound to the gastric mucus glycoprotein of normal and cystic fibrosis patients. Biochem Biophys Res Commun. 1983 May 31;113(1):286–293. doi: 10.1016/0006-291x(83)90464-3. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Wesley A., Mantle M., Man D., Qureshi R., Forstner G., Forstner J. Neutral and acidic species of human intestinal mucin. Evidence for different core peptides. J Biol Chem. 1985 Jul 5;260(13):7955–7959. [PubMed] [Google Scholar]
- Zanetta J. P., Breckenridge W. C., Vincendon G. Analysis of monosaccharides by gas-liquid chromatography of the O-methyl glycosides as trifluoroacetate derivatives. Application to glycoproteins and glycolipids. J Chromatogr. 1972 Jul 5;69(2):291–304. doi: 10.1016/s0021-9673(00)92897-8. [DOI] [PubMed] [Google Scholar]



