Abstract
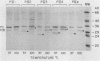
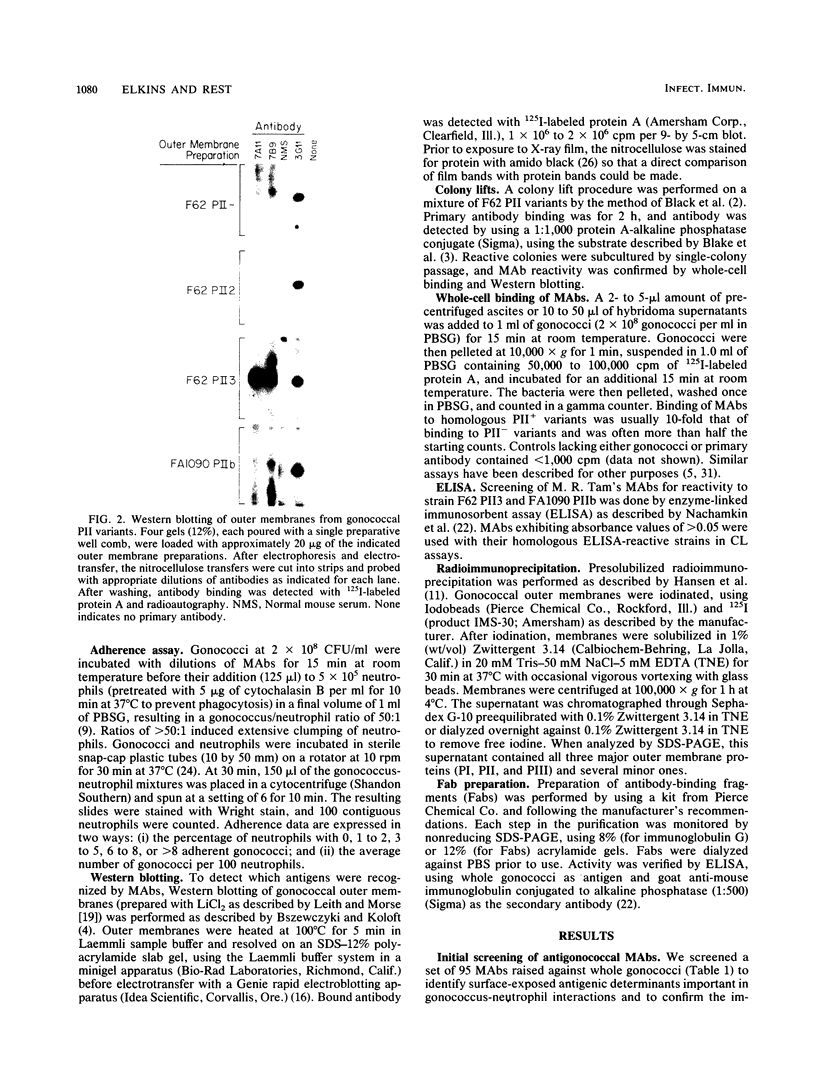
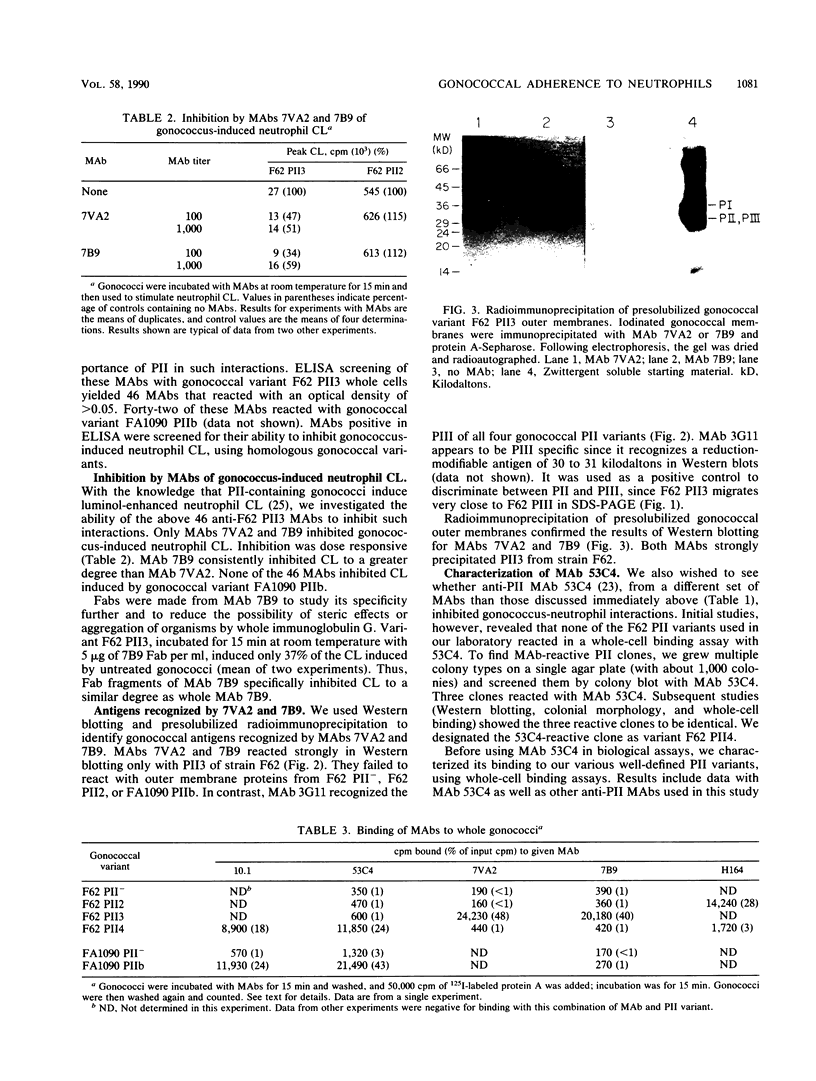
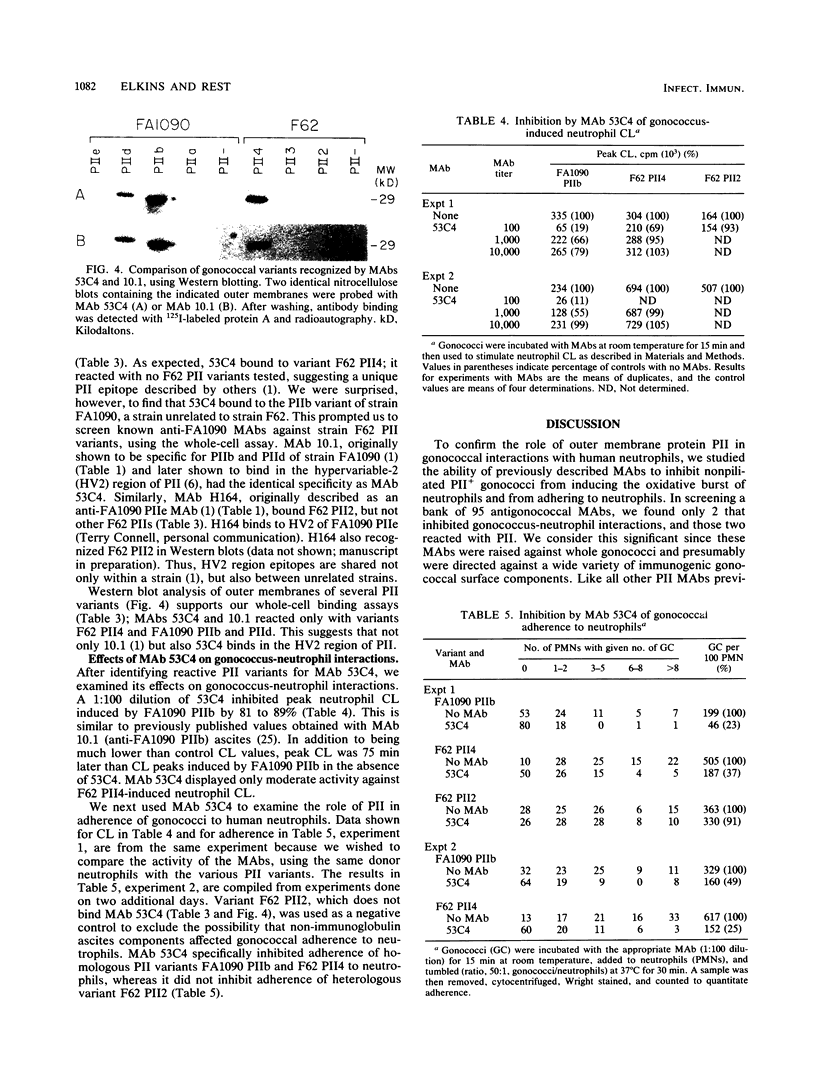
Nonopsonic binding of gonococci to human neutrophils appears to be mediated by a family of heat-modifiable outer membrane proteins termed protein IIs (PIIs). We studied the ability of a wide variety of antigonococcal monoclonal antibodies (MAbs) to inhibit the interactions of nonpiliated PII+ gonococci with human neutrophils by measuring gonococcal adherence to neutrophils and subsequent luminol-enhanced neutrophil chemiluminescence. From one set of 95 MAbs reacting with whole gonococci, only two, 7VA2 and 7B9, inhibited the ability of gonococci to induce neutrophil chemiluminescence. 7VA2 and 7B9 both reacted only with PII. MAb 53C4, from a smaller set of anti-PII MAbs, inhibited adherence to neutrophils of PII variants that bound 53C4, but not of PII variants that did not. It also inhibited gonococcus-induced neutrophil chemiluminescence. Using a whole-cell binding assay and Western blotting (immunoblotting), we showed that MAb 53C4 bound to one PII (PII4) of strain F62 and to two PIIs (PIIb and PIId) of strain FA1090. The present studies confirm and extend the role of PII in gonococcal adherence to and stimulation of human neutrophils and show intrastrain conservation of PII epitopes. The results indicate that PII is the only outer membrane component involved in adherence of nonpiliated gonococci to human neutrophils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barritt D. S., Schwalbe R. S., Klapper D. G., Cannon J. G. Antigenic and structural differences among six proteins II expressed by a single strain of Neisseria gonorrhoeae. Infect Immun. 1987 Sep;55(9):2026–2031. doi: 10.1128/iai.55.9.2026-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Schwalbe R. S., Nachamkin I., Cannon J. G. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984 Aug;45(2):453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Christensen K. K., Christensen P., Mårdh P. A., Weström L. Quantitation of serum antibodies to surface antigens of Neisseria gonorrhoeae with radiolabeled protein A of Staphylococcus aureus. J Infect Dis. 1976 Oct;134(4):317–323. doi: 10.1093/infdis/134.4.317. [DOI] [PubMed] [Google Scholar]
- Connell T. D., Black W. J., Kawula T. H., Barritt D. S., Dempsey J. A., Kverneland K., Jr, Stephenson A., Schepart B. S., Murphy G. L., Cannon J. G. Recombination among protein II genes of Neisseria gonorrhoeae generates new coding sequences and increases structural variability in the protein II family. Mol Microbiol. 1988 Mar;2(2):227–236. doi: 10.1111/j.1365-2958.1988.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Fischer S. H., Rest R. F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988 Jun;56(6):1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D. L., Roberts R. B. The interaction in vitro between human polymorphonuclear leukocytes and Neisseria gonorrhoeae cultivated in the chick embryo. J Exp Med. 1975 Jan 1;141(1):155–171. doi: 10.1084/jem.141.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Structure and surface exposure of protein IIs of Neisseria gonorrhoeae JS3. Infect Immun. 1985 May;48(2):452–457. doi: 10.1128/iai.48.2.452-457.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammel C. J., Sweet R. L., Rice P. A., Knapp J. S., Schoolnik G. K., Heilbron D. C., Brooks G. F. Antibody-antigen specificity in the immune response to infection with Neisseria gonorrhoeae. J Infect Dis. 1985 Nov;152(5):990–1001. doi: 10.1093/infdis/152.5.990. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Mayer L. W. Rates in vitro changes of gonococcal colony opacity phenotypes. Infect Immun. 1982 Aug;37(2):481–485. doi: 10.1128/iai.37.2.481-485.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. L., Connell T. D., Barritt D. S., Koomey M., Cannon J. G. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989 Feb 24;56(4):539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Cannon J. G., Mittler R. S. Monoclonal antibodies against Neisseria gonorrhoeae: production of antibodies directed against a strain-specific cell surface antigen. Infect Immun. 1981 May;32(2):641–648. doi: 10.1128/iai.32.2.641-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall WJ5th, Mail L. B., Wilde C. E., 3rd, Jones R. B. Purification and antigenic relatedness of proteins II of Neisseria gonorrhoeae. Infect Immun. 1985 Sep;49(3):576–580. doi: 10.1128/iai.49.3.576-580.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Fischer S. H., Ingham Z. Z., Jones J. F. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982 May;36(2):737–744. doi: 10.1128/iai.36.2.737-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Lee N., Bowden C. Stimulation of human leukocytes by protein II+ gonococci is mediated by lectin-like gonococcal components. Infect Immun. 1985 Oct;50(1):116–122. doi: 10.1128/iai.50.1.116-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. N., Jr, Clemens C. M., McGee Z. A., Cannon J. G. Immunoelectron microscopic localization of outer membrane proteins II on the surface of Neisseria gonorrhoeae. Infect Immun. 1988 Apr;56(4):1003–1006. doi: 10.1128/iai.56.4.1003-1006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Rest R. F. Interactions of gonococci with phagocytic cells. Annu Rev Microbiol. 1989;43:121–145. doi: 10.1146/annurev.mi.43.100189.001005. [DOI] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Sugasawara R. J., Cannon J. G., Black W. J., Nachamkin I., Sweet R. L., Brooks G. F. Inhibition of Neisseria gonorrhoeae attachment to HeLa cells with monoclonal antibody directed against a protein II. Infect Immun. 1983 Dec;42(3):980–985. doi: 10.1128/iai.42.3.980-985.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. 125I-labeled peptide mapping of some heat-modifiable proteins of the gonococcal outer membrane. Infect Immun. 1980 Apr;28(1):54–64. doi: 10.1128/iai.28.1.54-64.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Barrera O. Immunological characteristics of gonococcal outer membrane protein II assessed by immunoprecipitation, immunoblotting, and coagglutination. J Exp Med. 1983 May 1;157(5):1405–1420. doi: 10.1084/jem.157.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Gonococcal adherence: selected topics. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S678–S684. doi: 10.1093/clinids/5.supplement_4.s678. [DOI] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B., Kozloff L. M. A method for the efficient blotting of strongly basic proteins from sodium dodecyl sulfate-polyacrylamide gels to nitrocellulose. Anal Biochem. 1985 Nov 1;150(2):403–407. doi: 10.1016/0003-2697(85)90528-7. [DOI] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986 Feb;132(2):503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- Zak K., Diaz J. L., Jackson D., Heckels J. E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984 Feb;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]