Abstract
Although several autoimmune diseases are known to develop in postmenopausal women, the mechanisms by which estrogen deficiency influences autoimmunity remain unclear. Recently, we found that retinoblastoma-associated protein 48 (RbAp48) induces tissue-specific apoptosis in the exocrine glands depending on the level of estrogen deficiency. In this study, we report that transgenic (Tg) expression of RbAp48 resulted in the development of autoimmune exocrinopathy resembling Sjögren's syndrome. CD4+ T cell–mediated autoimmune lesions were aggravated with age, in association with autoantibody productions. Surprisingly, we obtained evidence that salivary and lacrimal epithelial cells can produce interferon-γ (IFN-γ) in addition to interleukin-18, which activates IFN regulatory factor-1 and class II transactivator. Indeed, autoimmune lesions in Rag2−/− mice were induced by the adoptive transfer of lymph node T cells from RbAp48-Tg mice. These results indicate a novel immunocompetent role of epithelial cells that can produce IFN-γ, resulting in loss of local tolerance before developing gender-based autoimmunity.
Autoimmune disease is controlled by environments that include gene variants or various cytokines (1, 2). It can increase susceptibility to autoimmunity by affecting the overall reactivity and quality of the cells of the immune system. There is an autoimmune disease specific for certain organs in the body, involving a response to an antigen expressed only in those organs. Antigen/organ specificity is affected by antigen presentation and recognition, antigen expression, and the state and response of the target organs (3, 4), which are maintained by a local immune system termed here “local tolerance.”
Many mechanisms protect tissues from autoimmune damage. These include relative isolation from the immune system and inhibition of the function of invading lymphocytes. For example, the eye has barriers to T cell infiltration and produces immunosuppressive cytokines, such as TGF-β (5). Constitutive expression of Fas ligand within the privileged site might also prevent immune-mediated damage by eliminating Fas-expressing T cells (6). Although they have yet to be well demonstrated in spontaneous animal models or human disease, genetic effects at the level of tissue protection are therefore to be expected. Autoimmune organ damage can be mediated by CD4+ T cells, which play a crucial role in the development of autoimmunity (7–9). MHC class II alleles are probably involved in autoimmune disease because different alleles have different abilities to present peptides from target cells to autoreactive CD4+ T cells (10, 11). Certain class II alleles might predispose to autoimmunity by increasing positive selection or decreasing negative selection of autoreactive T cells in the thymus. They might also act by inhibiting selection in the thymus of the regulatory CD4+ T cells that are thought to prevent autoantigen-specific responses. Evidence for the local tolerance hypothesis is provided by the observation that autoimmune diseases are often tissue specific and sometimes involve antibodies against a restricted set of antigens, thereby prompting us to accept this most simple explanation for the initiation of autoimmunity. The loss of local tolerance is considered to result from the combined effect of different environmental factors. MHC class II genes are constitutively expressed only on hematopoietic cells involved in antigen presentation (dendritic cells, macrophages, B cells, and cortical thymic epithelial cells), but can be aberrantly induced by inflammatory stimuli on many other cell types (such as endothelial cells, hepatocytes, β cells of the pancreas, and thyrocytes) (12, 13). Although it has been implicated in allograft rejection (14), and subsequently in autoimmunity, it is still unknown whether to initiate autoimmunity class II molecules have to be expressed on professional APCs within secondary lymphoid organs or on nonhematopoietic cells of the target organ itself.
It has been suggested that estrogenic action is responsible for the strong female preponderance of many autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren's syndrome (SS) (15, 16). Recent evidence suggests that apoptosis plays a key role in the physiology and pathogenesis of various autoimmune diseases, including SS (17–21). We have demonstrated that estrogenic action influences target epithelial cells through Fas-mediated apoptosis in a murine model for SS (21). Recently, we found that tissue-specific apoptosis in the exocrine glands spontaneously occurring in estrogen-deficient mice may contribute to the development of autoimmune exocrinopathy (22). Searching for the role of estrogen deficiency in the development of autoimmunity, we have recently identified retinoblastoma-associated protein 48 (RbAp48) gene specific for estrogen deficiency–dependent apoptosis in the exocrine glands, and transgenic expression of RbAp48 gene induced tissue-specific apoptosis in the exocrine glands (23). In this transgenic mouse model, we propose a possible clear and defined ab initio relationship between aberrant exposure of MHC class II molecules on IFN-γ–producing epithelial cells and disease development (i.e., autoimmune exocrinopathy).
RESULTS
Autoimmune exocrinopathy develops in RbAp48-transgenic (Tg) mice
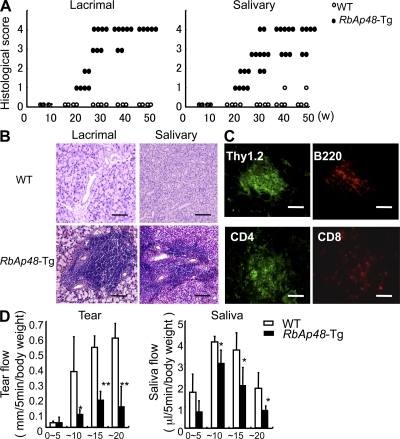
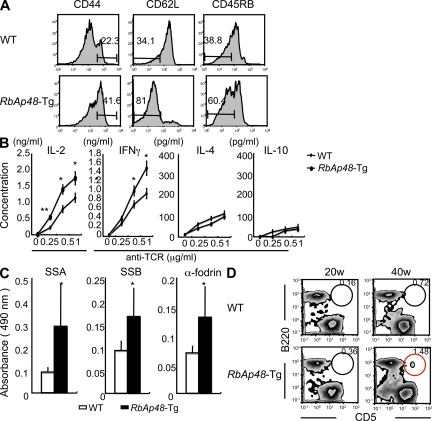
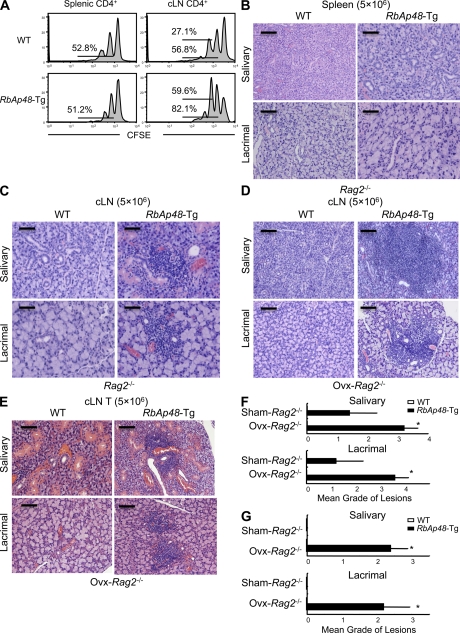
We have generated RbAp48-Tg mice where the RbAp48 gene is expressed in the salivary and lacrimal glands using the salivary gland–specific promoter (23). When the histopathology of all organs from those mice were analyzed, we found that autoimmune exocrinopathy resembling SS developed in almost all RbAp48-Tg mice at 24 wk of age or more, but not in the WT mice. Lymphocyte infiltration in salivary and lacrimal glands of RbAp48-Tg mice becomes more frequent at ∼30–50 wk of age (Fig. 1 A), and a significantly higher incidence of inflammatory lesions was found in female Tg mice at all ages (not depicted). Many infiltrating lymphocytes were observed in periductal areas at moderate (score 2) to severe (score 4) degrees, and shown in focal appearance. Representative histopathological features of the inflammatory lesions in lachrymal and salivary (submandibular) glands from RbAp48-Tg mice were shown in Fig. 1 B. No inflammatory lesions were observed in other organs of RbAp48-Tg mice. A majority of infiltrating cells in salivary and lacrimal glands were Thy1.2+ CD4+ T cells, whereas a minor proportion of B220+ B cells, CD8+ T cells (Fig. 1 C), and CD11b+ cells (unpublished data) was observed. When the function of lacrimal and salivary glands in RbAp48-Tg mice was analyzed, the mean volume of tear and saliva secretion from RbAp48-Tg mice was significantly lower than that from the WT group at 30 wk of age or more (Fig. 1 D). Regarding the peripheral T cell phenotype of RbAp48-Tg mice, T cell activation markers (CD44high, CD62Llow, CD45RBlow) were up-regulated on CD4+ T cells in cervical LNs (cLNs) from RbAp48-Tg mice, compared with those from WT mice (Fig. 2 A). No significant difference was observed in thymic T cells gated on CD4+CD8− bearing CD69, CD25, and CD62Llow between Tg and WT mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20080174/DC1). As for the phenotype of CD4+CD25+Foxp3+ T reg cells, no difference was detected in thymus, spleen, and cLNs between RbAp48-Tg and WT mice (Fig. S2). Moreover, culture supernatants from anti–TCR-β and -CD28 mAb-stimulated cLN T cells obtained from RbAp48-Tg mice contained higher levels of IL-2 and IFN-γ, whereas no difference in IL-4 and -10 levels between RbAp48-Tg and WT mice was observed by ELISA (Fig. 2 B). Our previous reports identified a 120-kD α-fodrin as an important autoantigen in murine and human SS (24, 25). It is particularly interesting that a higher titer of serum autoantibodies against SS-A (Ro), SS-B (La), and 120-kD α-fodrin was detected in RbAp48-Tg mice, compared with that in WT mice by ELISA (Fig. 2 C). This result is consistent with the characteristic flow cytometric finding that showed a significant CD5+B220+ fraction capable of autoantibody production (26) that appeared in spleen cells from RbAp48-Tg mice compared with WT mice (Fig. 2 D). On the other hand, the CD5+B220+ cells were undetectable in both salivary and lacrimal glands from RbAp48-Tg mice (Fig. S3 A). In addition, significantly increased CD21highIgMhighB220+ marginal zone B cells were observed in both cervical lymph nodes and spleen from RbAp48-Tg mice compared with those from WT mice (Fig. S3). These results may provide a new animal model for autoimmune exocrinopathy resembling SS, which should help us to further understand how autoreactive T cells are developed, and subsequently influence the development of autoimmunity.
Figure 1.
Autoimmune lesions in RbAp48-Tg mice. (A) Mean grade of inflammatory lesions in salivary and lacrimal glands from WT and RbAp48-Tg mice 10–50 wk of age. (B) Images (H&E staining) are representative of 5–7 mice at 32 wk of age. (C) Lymphocyte populations of the salivary gland lesion from RbAp48-Tg mice at 24 wk of age. Thy1.2+, CD4+, CD8+ T cells, or B220+ B cells were detected by immunofluorescence staining with FITC- (green) or PE-conjugated (red) mAbs using the frozen sections. Images are representative of three to five samples from each group. (D) The mean volume of saliva and tear secretion from WT and RbAp48-Tg mice at 30 wk of age was measured. Data are means ± SE of five mice. The results are representative of two independent experiments. Bars: (B) 100 μm; (C) 40 μm.
Figure 2.
Immune responses in RbAp48-Tg mice. (A and B) Activation markers and cytokine production of CD4+ T cells of cLNs from WT and RbAp48-Tg mice at 32 wk of age. (C) Autoantibodies of sera from RbAp48-Tg mice at 28–32 wk of age. (D) CD5+B220+ population of spleen from RbAp48-Tg and WT mice at 20 and 40 wk of age was analyzed by flow cytometry. Results are representative of means ± SE of five to seven mice in three independent experiments. *, P < 0.05; **, P < 0.005; WT versus RbAp48-Tg mice.
Salivary gland epithelial cells function as APCs
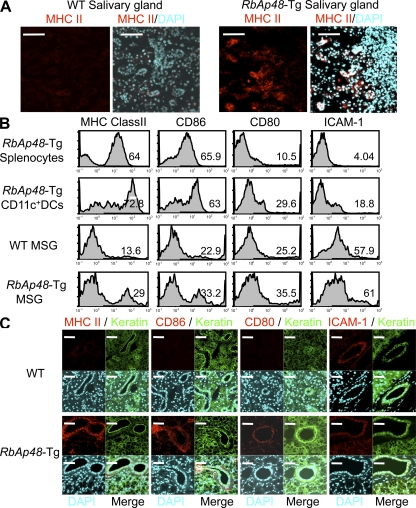
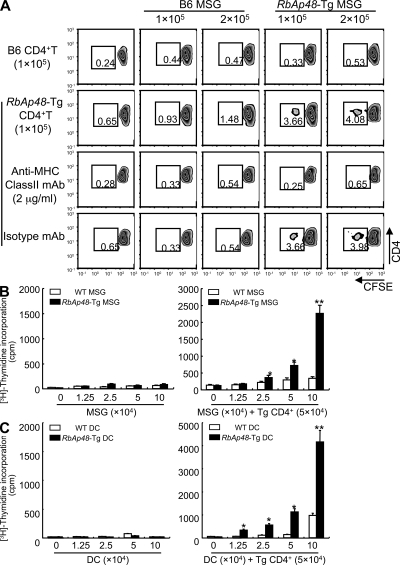
It is well known that nonlymphoid cells that express MHC class II molecules provoke autoimmune responses (12, 13). However, it is undetermined whether MHC class II–expressing epithelial cells can function as APCs. We frequently observed MHC class II molecule expression on the exocrine gland cells in RbAp48-Tg mice, but not in WT mice (Fig. 3 A). These molecules play a pivotal role in the induction and regulation of immune responses by virtue of their ability to present self-peptides to CD4+ T cells (27). To examine whether salivary epithelial cells could act as APCs, mouse salivary gland (MSG) cells, splenocytes, and splenic CD11c+ DCs from RbAp48-Tg mice and WT mice were compared in terms of their capacity to express MHC class II and costimulatory molecules, including CD86, CD80, and ICAM-1, by flow cytometric analysis. Among them, a considerably large proportion of MHC class II+, CD86+ cells, CD80+ cells, and ICAM-1+ cells was observed on MSG cells from Tg mice, compared with those from WT mice (Fig. 3 B). MSG cells were enriched by enzymatic treatment and using several antibodies against immune cells, epithelial cells, and magnetic beads, as shown in Fig. S4 A (available at http://www.jem.org/cgi/content/full/jem.20080174/DC1). DCs were undetectable in the purified MSG cell suspension (Fig. S4 B). On the other hand, although the expressions of MHC class II and CD86 on the splenocytes and CD11c+ DCs were higher than those on both MSG cells, the expressions of CD80 and ICAM-1 on the professional APCs were similar or lower than those on the MSG cells (Fig. 3 B). These expressions (MHC class II+, CD86+, CD80+, and ICAM-1+) on salivary epithelial cells from RbAp48-Tg mice were also confirmed by confocal analysis (Fig. 3 C). Controls using isotype antibodies for immunostainings were shown in Fig. S4 B. Moreover, to examine whether the peripheral T cells from RbAp48-Tg mice can respond to the MSG cells that show phenotypes for the APCs, CFSE-labeled purified CD4+ (105) T cells from RbAp48-Tg mice were co-cultured with the MSG cells (1 and 2 × 105) from those mice or WT mice. Although CD4+ T cells from B6 mice could not respond to both B6 and RbAp48-Tg MSG cells, CD4+ T cells from RbAp48-Tg mice were capable of responding to MSG cells from RbAp48-Tg mice, but not with those from WT mice, whereas anti–MHC class II antibody inhibits these responses (Fig. 4 A). Furthermore, proliferation assay using [3H]thymidine incorporation demonstrated that purified CD4+ T cells of cLNs from RbAp48-Tg mice were more proliferative to the MSG cells from RbAp48-Tg mice relative to those from WT mice (Fig. 4 B). Additionally, significantly increased proliferation of the CD4+ T cells to peripheral DCs from RbAp48-Tg mice was observed compared with DCs from WT mice (Fig. 4 C). Approximately half of the T cells response to 105 DCs from RbAp48-Tg mice equaled the level of the response to 105 MSG cells from the Tg mice (Fig. 4, B and C).
Figure 3.
Antigen-presenting function in salivary epithelial cells. (A) MHC class II expression of salivary tissues from WT and RbAp48-Tg mice was detected by confocal analysis using anti–MHC class II mAb, Alexa Fluor 568–conjugated anti–rat IgG (red) and DAPI (blue). Images are representative of five to seven mice. (B) APC markers of splenocytes, splenic DCs, and MSG cells from WT and RbAp48-Tg mice were analyzed by flow cytometer. Results are representative of three to five mice in two independent experiments. (C) APC markers of MSG epithelial cells were detected by confocal analysis using anti-MHC class II, CD86, CD80, ICAM-1 mAbs, anti-keratin polyclonal antibody, Alexa Fluor 568–conjugated anti–rat IgG (red), Alexa Fluor 488–conjugated anti–rabbit IgG (green), and DAPI (blue). Images are representative of five to seven mice. Bars: (A and C) 50 μm.
Figure 4.
CD4+ T cells can proliferate to epithelial cells from RbAp48-Tg mice. (A) CFSE-labeled purified CD4+ T cells (105) of WT and RbAp48-Tg mice were co-cultured with MSG cells (1 and 2 × 105) from the mice for 72 h. Cell proliferation was estimated by the dilution of CFSE. 2 μg/ml anti–MHC class II mAb or isotype control antibody was added in the culture. All results are representative of three to five mice at 28 wk of age, or three-independent experiments. (B) CD4+ T cells (5 × 104) of cLNs from RbAp48-Tg mice were co-cultured with irradiated MSG cells (0–10 × 104) from WT and RbAp48-Tg mice for 72 h. (C) CD4+ T cells (5 × 104) of cLNs from RbAp48-Tg mice were co-cultured with irradiated DCs (0–10 × 104) from WT and RbAp48-Tg mice for 72 h. Proliferative T cell response was evaluated by [3H]thymidine incorporation during the last 12 h of the culture. Results are representative of means ± SE of triplicates in two independent experiments. *, P < 0.05; **, P < 0.005; WT versus RbAp48-Tg MSG cells or DCs.
Crucial role of epithelial IFN-γ production
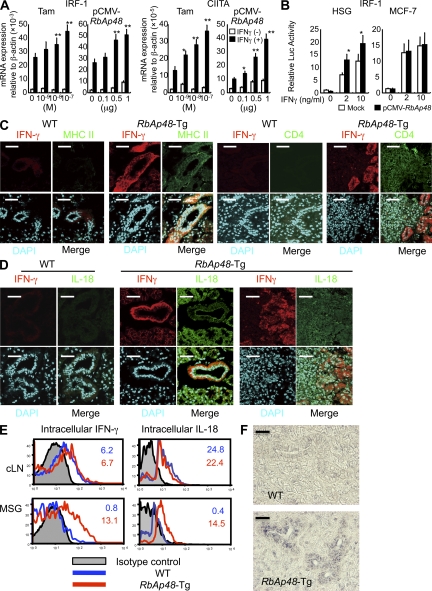
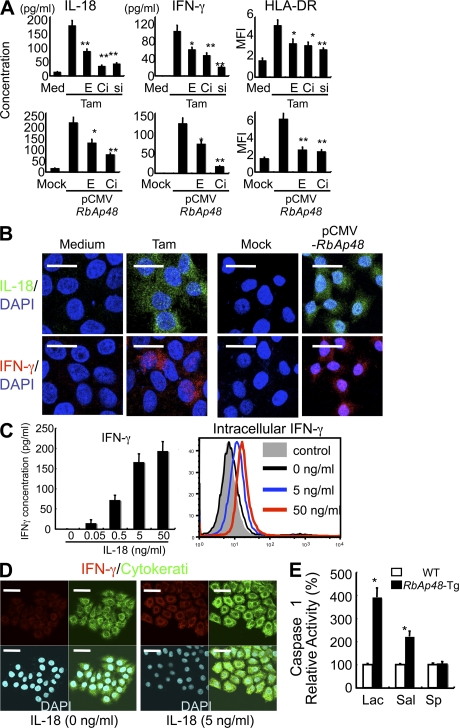
The expression of MHC class II molecules is generally regulated at the transcriptional levels, including the transcription factor IFN regulatory factor (IRF)-1 (28, 29) and the class II transactivator (CIITA), which is the master regulator for MHC class II gene expression (30, 31). It has been shown that IRF-1 is a primary responsible gene of the IFN-γ response (32). In in vitro studies using human salivary gland (HSG) cells (33), IFN-γ–induced mRNAs of IRF-1 and CIITA were significantly enhanced by treatment with Tamoxifen (Tam), which is an antagonist of estrogen and can induce RbAp48 (23), or transfection of pCMV-RbAp48 plasmid in the dose-dependent manner (Fig. 5 A), not in MCF-7 cells (human mammary gland cell line; Fig. S5, A and B, available at http://www.jem.org/cgi/content/full/jem.20080174/DC1). In addition, we next analyzed the IRF-1 promoter activity using RbAp48-transfected HSG cells with and without IFN-γ by luciferase assay. We observed significantly enhanced IRF-1 promoter activity in RbAp48-transfected HSG cells with IFN-γ, not in MCF-7 cells (Fig. 5 B). Surprisingly, in RbAp48-Tg mice, a prominent expression of IFN-γ was detected in salivary and lacrimal epithelial cells besides sporadically positive infiltrating cells of RbAp48-Tg mice, not WT mice (Fig. 5 C). These findings were observed mainly in the MHC class II+ ductal epithelium adjacent to lymphoid infiltrates. Epithelial IFN-γ expression in the exocrine glands of RbAp48-Tg mice was up-regulated during the course of autoimmune exocrinopathy. Induction of IFN-γ expression may occur through many different types of stimulation, including cross-linking of cell-surface receptors and stimulation with cytokines, including IL-2, -12, and -18 (34). It has been demonstrated that IFN-γ synthesis is predominantly induced by stimulation with IL-18 (35). Consistent with a previous study (36), IL-18 expression was observed in salivary epithelial cells in RbAp48-Tg mice, not in WT mice (Fig. 5 D). Confocal analysis revealed that differential expression of IL-18 and IFN-γ was clearly observed, i.e., IL-18 mainly in the acinar cells and IFN-γ in the duct cells, within salivary epithelial cells from RbAp48-Tg mice, but not from WT mice (Fig. 5 D). Controls using isotype antibodies were shown in Fig. S4 C. Epithelial IFN-γ and IL-18 productions were confirmed by flow cytometry using MSG cells without immune cells from RbAp48-Tg, not from WT mice, whereas there was no difference in both IFN-γ and IL-18 expressions of cLN cells between WT and RbAp48-Tg mice (Fig. 5 E). Although the production of IL-18 in salivary gland cells was detected in a previous study (36), there has been no proof for IFN-γ production of salivary gland cells in any paper. Therefore, to confirm IFN-γ production of exocrine glands, detection of IFN-γ using tissue homogenates was performed. A high concentration of IFN-γ was detected in the tissue homogenates of lacrimal and salivary glands from RbAp48-Tg mice, compared with that from WT mice, by ELISA (Fig. S6 A). Furthermore, the detection of IFN-γ mRNA of MSG cells was performed by in situ hybridization using the RNA probe of mouse IFN-γ gene. A more intense signal for IFN-γ mRNA in duct cells of salivary glands from RbAp48-Tg mice was observed compared with that from WT mice (Fig. 5 F). As for the expression of BAFF, which is an inducer of IFN-γ in B cells, the expression of epithelial cells was undetectable in both RbAp48-Tg and WT mice (Fig. S7). In vitro studies using HSG cells demonstrated that the expressions of IL-18, IFN-γ, and MHC class II (HLA-DR) were observed when treated with Tam or transfected with pCMV-RbAp48, whereas they were inhibited when treated with 17β-estradiol (E2), caspase 1 inhibitor (Ac-YVAD-CHO; Ci), and siRNA of RbAp48 (si; Fig. 6 A). Confocal analysis confirmed the expression of IL-18 and IFN-γ in HSG cells treated with Tam or transfected with pCMV-RbAp48 (Fig. 6 B). It is important to note that IL-18 is secreted earlier (by 6 h) than IFN-γ production and HLA-DR expression (by 12 h) in Tam-stimulated and RbAp48-transfected HSG cells (Fig. S8). The most prominent function of IL-18 is its capacity to act as a potent costimulus for IFN-γ production (37–39). Indeed, we observed an increase in IFN-γ production in HSG cells treated with recombinant IL-18 in the dose-dependent manner, but not in MCF-7 cells (Fig. S9, available at http://www.jem.org/cgi/content/full/jem.20080174/DC1), by ELISA and flow cytometry (Fig. 6 C). Confocal analysis of IFN-γ production of HSG cells in response to IL-18 together with cytokeratin as an identified marker was shown in Fig. 6 D. Moreover, we found a significant up-regulation of caspase 1 activity in lacrimal and salivary glands from RbAp48-Tg mice relative to that from WT mice (Fig. 6 E). In this regard, we reported previously significantly increased caspase 1 activity in salivary gland tissues from ovariectomized (Ovx) C57BL/6 mice in vivo (22) and Tam-stimulated and RbAp48-transfected HSG cells in vitro (23).
Figure 5.
IFN-γ production from salivary epithelial cells stimulated with RbAp48. (A) IRF-1 and CIITA mRNA expressions of HSG cells stimulated with Tam (∼10−9–10−7 M) or transfected with pCMV-RbAp48 (∼0–1 μg) in the presence of IFN-γ (5 ng/ml) were detected by real-time PCR. Data are shown as means ± SE (SE) relative to β-actin mRNA of two independent experiments. *, P < 0.05; **, P < 0.005, versus IFN-γ (+) Tam 0 M or IFN-γ (+) pCMV-RbAp48 0 μg. (B) Promoter activity of IRF-1 in HSG and MCF-7 cells transfected with pCMV-RbAp48. Data are shown as means ± SE (SE) of two independent experiments. *, P < 0.05; **, P < 0.005, versus Mock (C and D) Confocal analysis of IFN-γ, MHC Class II, CD4, IL-18 or DAPI of salivary gland tissues from WT and RbAp48-TG mice at 32 wk of age. Alexa Fluor 488– or Alexa Fluor 568–conjugated anti–rat IgG were used as the second antibodies. Images are representative of three to five mice. (E) Intracellular IFN-γ and IL-18 expressions of cLN and MSG (without immune cells) cells from WT and RbAp48-Tg mice at 32 wk of age was detected by flow cytometric analysis. Results are representative and are shown as means ± SE of three mice of each group in two independent experiments. (F) The expression of IFN-γ mRNA in salivary gland cells of RbAp48-Tg mice was detected by in situ hybridization. Representative images of WT and RbAp48-Tg mice are shown in two independent experiments. Negative (antisense probe) or positive controls for mouse IFN-γ RNA probe are shown in Fig. S6 B. Fig. S6 is available at http://www.jem.org/cgi/content/full/jem.20080174/DC1. Bars: (C) 50 μm; (D) 40 μm.
Figure 6.
Expressions of IL-18, IFN-γ, and MHC class II (HLA-DR) in HSG cells when treated with Tam and transfected with pCMV-RbAp48. (A) Inhibitory effects of 10−9 M 17β-estradiol (E) or 10 μM caspase 1 inhibitor (Ci) on RbAp48-induced IL-18, IFN-γ, and HLA-DR in HSG cells. IL-18 and IFN-γ of the culture supernatants were detected by ELISA. HLA-DR is shown as mean fluorescence intensity (MFI) by flow cytometric analysis. *, P < 0.05; **, P < 0.005, versus RbAp48-induced. Data are means ± SD of triplicate samples, and representative of two independent experiments. (B) Tam- or RbAp48-induced IL-18 and IFN-γ were detected by confocal microscopic analysis. IL-18, IFN-γ mAbs, and Alexa Fluor 488– or Alexa Fluor 568–conjugated anti–mouse IgG were used. Images are representative of three independent experiments. (C) IFN-γ secretion or production of HSG cells by the addition of recombinant IL-18 was detected by ELISA or intracellular flow cytometric analysis. Data are representative of three independent experiments. (D) IFN-γ expression of IL-18–stimulated HSG cells was detected by confocal microscopic analysis together with cytokeratin and DAPI stainings. Images are representative of three independent experiments. (E) Caspase 1 activity of lacrimal, salivary glands, and spleen from RbAp48-Tg mice at 28 wk of age. Data are shown as means ± SE of four mice in two independent experiments, relative to those of WT mice. *, P < 0.05; **, P < 0.005; WT versus RbAp48-Tg mice. Bars: (B) 20 μm; (D) 50 μm.
Transfer of autoimmune exocrinopathy in RbAp48-Tg mice
Because thymic T cell abnormality could not be observed in RbAp48-Tg mice, it is speculated that there may be dysregulation of peripheral tolerance. To know the homeostatic expansion of peripheral T cells from RbAp48-Tg mice, we adoptively transferred CFSE-labeled CD4+ T cells from RbAp48-Tg mice into irradiated syngeneic C57BL/6.Ly5.1 mice and analyzed them 7 d later. As a result, we observed more substantial cell division of the donor CFSE-labeled cLN CD4+ T cells from RbAp48-Tg mice than that from WT mice (Fig. 7 A), indicating that T cells undergoing homeostatic proliferation may provide a basis for autoimmunity (40, 41). This suggests that elicitation of CD4+ T cell–mediated autoreactivity against autoantigen could be the primary pathogenic process that leads to substantial homeostatic expansion. Futhermore, we succeeded in adoptive transfer of autoimmune lesions in the exocrine glands into Rag2−/− mice using cervical lymph node cells, but not spleen cells, from RbAp48-Tg mice (Fig. 7, B and C). Interestingly, these transferred lesions were extremely enhanced in estrogen-deficient Rag2−/− mice treated with ovariectomy (Ovx) compared with the lesions in Sham Rag2−/− mice (Fig. 7, D and F), suggesting that estrogen deficiency accelerates autoimmune exocrinopathy, as previously reported (21, 22). When we examined the adoptive transfer using T cells isolated from cervical lymph nodes and spleen of RbAp48-Tg mice, no inflammatory lesions had developed in Rag2−/− mice (Table I). These data suggest that APCs besides T cells might be required for successful transfer of autoimmune exocrinopathy in RbAp48-Tg mice. Finally, to confirm that MHC class II expression on activated salivary or lacrimal gland cells of RbAp48-Tg mice can drive priming of purified T cells of cLNs from RbAp48-Tg mice to induce autoimmune lesions, the T cells of cLNs from RbAp48-Tg mice were transferred into Ovx-Rag2−/− mice. The autoimmune lesions of salivary and lacrimal glands from the recipient Ovx-Rag2−/− mice transferred with T cells of cLNs from RbAp48-Tg mice were observed, whereas no lesions were found in any organs of the recipient Ovx-Rag2−/− mice transferred with T cells of cLNs from WT mice (Fig. 7, E and G). These results demonstrate that the epithelial cells stimulated through increased RbAp48 because of estrogen deficiency could interact with T cells to induce autoimmunity via loss of local tolerance.
Figure 7.
Transfer of autoimmune lesions from RbAp48-Tg mice into Rag2−/− mice. (A) Homeostatic proliferation of splenic and cLN T cells from WT and RbAp48-Tg mice at 28 wk of age was analyzed at 7 d after transfer into irradiated C57BL/6 mice. Results are representative of four to five mice in two independent experiments. Percentages of divided cells from the second or third division are indicated. (B and C) Spleen cells (5 × 106) or cLN cells (5 × 106) from WT and RbAp48-Tg mice at 30 wk of age were transferred into Rag2−/− mice. At 6 wk after the transfer, the pathology of salivary and lacrimal glands was analyzed. Images are representative of four to five mice. (D) cLN cells from WT and RbAp48-Tg mice were transferred into ovariectomized (Ovx) Rag2−/− mice. (E) T cells of cLNs from WT and RbAp48-Tg mice were transferred into ovariectomized (Ovx) Rag2−/− mice. Images of salivary and lacrimal gland tissues were representative of four to five mice. (F) Severity of inflammatory lesions of salivary and lacrimal glands from sham-operated (Sham) and Ovx-Rag2−/− hosts by transfer of cLN cells were shown as mean grade of lesions. (G) Severity of inflammatory lesions of salivary and lacrimal glands from sham-operated (Sham) and Ovx-Rag2−/− hosts by T cell transfer were shown as mean grade of lesions. Data are shown as means ± SE of four to five mice. *, P < 0.05, Sham versus Ovx. Bars: (B–E) 100 μm.
Table I.
Induction of autoimmune lesions
| Donor cells | Mice | Incidence |
|---|---|---|
| Spleen cells (5×106) | WT | 0/4 |
| RbAp48-Tg | 0/4 | |
| cLN cells (5×106) | WT | 0/5 |
| RbAp48-Tg | 4/5 | |
| cLN T cells (5×106) | WT | 0/4 |
| RbAp48-Tg | 0/5 | |
| cLN B cells (5×106) | WT | 0/4 |
| RbAp48-Tg | 0/4 |
Whole spleen, cLN cells, cLN T cells, or cLN B cells were transferred intravenously into Rag2−/− mice. The host mice were killed 6 wk after transfer. Inflammatory lesions of salivary or lacrimal glands were evaluated by pathological analysis.
IFN-γ and IL-18 expressions in human SS patients
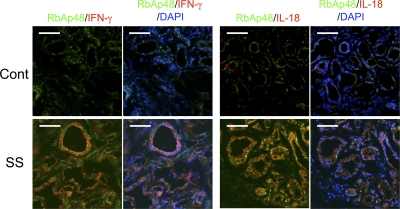
Although it has been reported that immune cells express some cytokines, it is unclear whether IFN-γ or IL-18 together with RbAp48 in the epithelial cells of salivary glands from human SS patients are expressed. To confirm our hypothesis that autoimmunity is induced by a breakdown of local tolerance in salivary gland cells with up-regulated RbAp48 because of estrogen deficiency such as menopause, IFN-γ, IL-18, and RbAp48 expressions were detected by confocal microscopic analysis using human biopsy samples from SS patients and controls. Among 10 SS patients, RbAp48+ and IFN-γ+ epithelial cells were observed in three samples and RbAp48+ and IL-18+ epithelial cells were observed in four samples. A representative image of SS patients and controls is shown in Fig. 8. Although faint expressions of RbAp48 in the nucleus of salivary epithelial cells were detected in control samples, IL-18 or IFN-γ together with a prominent expression of RbAp48 was not observed (Fig. 8). Isotype-matched controls of staining for the mAbs were shown in Fig. S10 (available at http://www.jem.org/cgi/content/full/jem.20080174/DC1).
Figure 8.
IFN-γ and IL-18 expressions together with RbAp48 in salivary glands from SS patients. The frozen sections of salivary glands from SS patients and controls were stained with IFN-γ or IL-18 (Alexa Fluor 568; red) and RbAp48 (Alexa Fluor 488; green) mAbs. The nuclei were stained with DAPI. The representative images in controls and SS patients were shown in three independent experiments.
DISCUSSION
Although MHC class II molecules have been expressed aberrantly on epithelial cells in association with autoimmunity, it remains debatable whether class II molecules are the initiating event or the consequence of the autoimmune attack. For example, certain alleles of class II (mouse I-Ag7) might be particularly good at presenting glutamic acid decarboxylase–65 or insulin peptides to T cells in nonobese diabetic (NOD) mice, thus contributing to recognition and ultimate destruction of pancreatic β cells (10, 11). Some investigators have proposed that I-Ag7, because of its poor peptide-binding properties, enhances autoimmunity in NOD mice in a global fashion (42). In this case, the β cell specificity of autoimmunity in this strain and the switch to autoimmune thyroiditis when a class II molecule without these properties is exchanged for I-Ag7 must be controlled by other genetic loci in NOD mice (43). It is possible that the most straightforward explanation for the effects of I-Ag7 is that it predisposes to islet-specific autoimmunity and not system-wide reactivity. Another piece of evidence that the role of MHC class II is antigen-specific is that the class II alleles predisposing toward autoimmunity vary in one human disease to another, indicating that class II alleles act in autoimmunity via specific antigens rather than comprehensively.
We demonstrated in this study that autoimmune exocrinopathy resembling SS developed in almost all RbAp48-Tg mice, and that a high titer of serum autoantibodies against SS-A (Ro), SS-B (La), and 120-kD α-fodrin was detected in these Tg mice. We frequently found MHC class II molecule expression on the exocrine gland cells with autoimmune lesions in RbAp48-Tg mice. When we examined whether salivary epithelial cells could act on antigen presentation, we found a large proportion of MHC class II+, CD86+, CD80+, and ICAM-1+ cells primarily observed on cultured MSG cells from Tg mice. Moreover, CFSE-labeled purified CD4+ (105) T cells from RbAp48-Tg mice were capable of responding to MSG cells from RbAp48-Tg mice, whereas anti–MHC class II antibody inhibited these responses. Although it has not been determined whether MHC class II–expressing epithelial cells can function as APCs, those data strongly suggest that the epithelial cells may function as APCs during development of autoimmune exocrinopathy. In RbAp48-Tg mice, a surprisingly prominent expression of epithelial IFN-γ was detected beside sporadically positive infiltrating cells. These findings were observed mainly in the MHC class II+ ductal epithelium adjacent to lymphoid infiltrates. Epithelial IFN-γ expression in the exocrine glands of RbAp48-Tg mice was up-regulated during the course of autoimmune exocrinopathy. A previously unknown, multifaceted role of IFN-γ as regulator of the local immune system, which is termed here local tolerance, is disclosed. As to the mechanism of CIITA induction in RbAp48-Tg mice, our findings demonstrate the essential role of RbAp48-driven stimulation of IFN-γ production and signaling leading to up-regulation of IRF-1 and CIITA. RbAp48, initially identified as retinoblastoma-binding proteins (44), was characterized as a component of distinct nucleosome-modifying complexes, including the nuclear histone deacetylases (45, 46). Although the functions of the RbAp48-like proteins in these complexes remain undetermined, it was reported that E2F-1 and RbAp48 are physically associated in the presence of Rb and histone deacetylase (47), suggesting that RbAp48 could be involved in transcriptional repression of E2F-responsive genes. Several reports have demonstrated that estrogen may play an inhibitory role on apoptosis in endothelial cells, breast cancer cells, cardiac myocytes, prostate cells, and neuronal cells (48–51). It has been shown that the transcription factor IRF-1 mRNA expression is induced by ICI 182,780 as an antiestrogenic reagent and repressed by estrogens in antiestrogen-sensitive cells (52). We demonstrated the first evidence that IFN-γ–producing epithelial cells in the exocrine glands function as APCs through the IRF-1–CIITA pathway, resulting in the development of autoimmune exocrinopathy via loss of local tolerance. SS is known to have the most female predominance of >95% among all the autoimmune patients (15, 16). One of the key questions in respect to the pathophysiology of autoimmune diseases is how autoreactivity to particular autoantigens is initiated and maintained under an estrogen-deficient state. Although an important role for T cells in the development of autoimmune disease has been argued (53, 54), it is not known if disease is initiated by a retrained inflammatory reaction to autoantigen. We clarified that epithelial IFN-γ production is crucial for the initiation of autoimmune reactions between epithelial cells and autoreactive T cells with homeostatic expansion. Our previous study suggests that antiestrogenic actions have a potent effect on the proteolysis of α-fodrin autoantigen in the salivary gland through up-regulation of caspase 1 activity (22). These results strongly suggest that RbAp48-mediated activation of caspase 1 leads to the cleavage and activation of IL-18, which may act directly on IFN-γ production and on effector CD4+ T cells by inducing migration and proliferation. Thus, aberrant expression of MHC class II in the exocrine glands facilitates loss of local tolerance before the development of autoimmune lesions, which is very similar to SS.
Evidence has been mounting that estrogen deficiency such as menopause is a proinflammatory state, which promotes osteoporosis and atherosclerosis, as well as autoimmunity (55, 56). In vivo and in vitro experiments here, including the induction of autoimmune lesions by T cell transfer from RbAp48-Tg mice into Ovx-Rag2−/− mice and in vitro antigen presentation of the Tg MSG cells to CD4+ T cells, strongly suggests that estrogen deficiency stimulates salivary epithelial cells that are activated via the up-regulation of RbAp48 to present any endogenous autoantigen to CD4+ T cells for the onset of autoimmune lesion in the salivary glands resembling human SS. Our data finds that transfer of cLN T cells from RbAp48-Tg mice into Ovx-Rag2−/− mice leads to autoimmune lesions, consistent with the conclusion that estrogen deficiency leads to the ability of salivary gland epithelial cells to express MHC class II and present any self-antigens. Most importantly, the salivary gland cells from human SS patients express RbAp48 together with IFN-γ or IL-18, as well as the findings of RbAp48-Tg mice in this work, suggesting that the molecules would be useful for any clinical application.
Collectively, our results demonstrate a direct molecular mechanism by which estrogen deficiency induces tissue-specific overexpression of RbAp48 (23), subsequently developing CD4+ T cell–mediated autoimmunity through epithelial IFN-γ production. Thus, reducing the RbAp48 overexpression is a possible effective therapy in gender-based autoimmune exocrinopathy.
MATERIALS AND METHODS
Mice and histology.
RbAp48-Tg mice have been previously described (23), and the RbAp48 gene is regulated by lacrimal and salivary gland–specific promoter (57). Rag2−/− mice were obtained from Taconic. All mice were reared in our specific pathogen–free mouse colony, and given food and water ad libitum. The experiments were approved by an animal ethics board of Tokushima University. All organs were removed from the mice, fixed with 4% phosphate-buffered formaldehyde (pH 7.2), and prepared for histological examination. Formalin-fixed tissue sections were subjected to hematoxylin and eosin (H&E) staining, and three pathologists independently evaluated the histology without being informed of the condition of each individual mouse. Histological grading of the inflammatory lesions was done according to the method proposed by White and Casarett (58), as follows: 1 = 1–5 foci composed of >20 mononuclear cells per focus, 2 = >5 such foci, but without significant parenchymal destruction, 3 = degeneration of parenchymal tissue, and 4 = extensive infiltration of the glands with mononuclear cells and extensive parenchymal destruction. Histological evaluation of the salivary and lacrimal glands was performed in a blind manner, and a tissue section from each salivary and lacrimal gland was examined.
Measurement of fluid secretion.
Analysis of the tear and saliva volume of WT and RbAp48-Tg mice was performed according to a previously described method (59).
Flow cytometric analysis.
Lymphocytes in spleen, cLN, thymus, and MSG epithelial cells without immune cells (>95% of cells were keratin+) were prepared. Surface markers were identified by mAbs with an EPICS flow cytometer (Beckman Coulter). Rat mAbs to FITC-, PE-, or PE-Cy5–conjugated anti-B220, CD4, MHC class II, CD86, CD80, ICAM1, and CD5 mAbs (eBioscience) were used. Appropriate isotype-matched controls were used, respectively. For detection of T cell activation markers, FITC-conjugated anti-CD25, CD44, CD62L, CD45RB, and CD69 mAbs (eBioscience) were used. Intracellular Foxp3 expression with an Intracellular Foxp3 Detection kit (eBioscience) was performed according to the manufacturer's instructions. Detections of intracellular IFN-γ or IL-18 were also performed by the same procedure. The data were analyzed with FlowJo FACS Analysis software (Tree Star, Inc.).
ELISA.
The amount of mouse IL-2, IFN-γ, IL-4, and IL-10 in culture supernatants from CD4+ T cells stimulated with anti-TCR mAb (∼0–1 μg/ml) and anti-CD28 mAb (20 μg/ml; eBioscience), anti-SSA, anti-SSB, and anti–α-fodrin autoantibodies of sera from WT and RbAp48-Tg mice and human IL-18 and IFN-γ from cultured HSG and MCF-7 cells were analyzed by ELISA. In brief, plates were coated with a capture antibody or recombinant proteins (SSA, SSB, and α-fodrin), and washed with PBS/0.1% Tween 20. The plates were incubated with diluted culture supernatants or sera. After washing with PBS/0.1% Tween 20 and incubation of biotin-conjugated antibodies for cytokine detection, a horseradish peroxidase–conjugated detection antibody for autoantibody detection was added. After incubation with streptavidin-horseradish peroxidase for cytokine detection, plates were again washed with PBS/0.1% Tween 20 and o-phenylendiamine (Sigma-Aldrich) buffer added. Plates were then analyzed with a microplate reader reading at 490 nm.
Confocal microscopic analysis.
Confocal microscopic analysis using anti-Thy1.2, B220, CD4, CD8, MHC class II, IFN-γ, CD4 (eBioscience), keratin (LSL CO., LTD), and IL-18 (MBL) antibodies was performed on the frozen sections of salivary glands from WT and RbAp48-Tg mice, and on the cultured cells using Confocal Laser Microscan (LSM 5 PASCAL; Carl Zeiss, Inc.). As the second antibodies, Alexa Fluor 488 anti–mouse IgG (H+L), Alexa Fluor 568 goat anti–rabbit IgG (H+L), Alexa Fluor 488 donkey anti–rat IgG (H+L), Alexa Fluor 488 chicken anti–goat IgG (H+L), and Alexa Fluor 568 rabbit anti–goat IgG (H+L; Invitrogen) were used. The nuclear DNA was stained with DAPI (Invitrogen).
Cell culture.
For the co-culture of MSG with CD4+ T cells, MSG cells were prepared by digestion of collagenase and hyaluronidase, and CD11c+, CD11b+, B220+, NK1.1+, and Thy1.2+ cells were removed by the mAbs and magnetic bead–conjugated anti–rat IgG (Invitrogen). CD4+ T cells from cLNs were purified by mAbs (anti–MHC class II, CD8, CD11b, CD11c, B220, and NK1.1) and magnetic bead–conjugated anti–rat IgG. CFSE-labeled CD4+ T cells were co-cultured with MSG cells for 72 h. Cell division of CD4+ T cells was analyzed by dilution of CFSE through flow cytometry. As for the co-culture with DCs or MSG cells, DCs from RbAp48-Tg or WT mice were enriched using DC collection kit (Invitrogen). After DCs or MSG cells were irradiated (9 Gy), purified CD4+ T cells of cLNs from RbAp48-Tg mice were co-cultured with the DCs or MSG cells for 72 h. The T cells were then pulsed with 0.5 μCi [3H]thymidine per well for the last 12 h of the culture. [3H]thymidine incorporation was evaluated using an automated β liquid scintillation counter. HSG and MCF-7 cells were cultured in DME containing 10% FBS at 37°C. Tam (Wako Pure Chemical), 17β-estradiol (Wako), 10 μM caspase 1 inhibitor (Sigma-Aldrich), and recombinant human IFN-γ (R&D Systems) were used for cell cultures. RbAp48 gene inserted into pCMV (2N3T) construct (a gift from D. Trouche, Centre National de la Recherche Scientifique, University of Tolouse, Tolouse, France) (47) was transfected into the cells using FuGENE6 Transfection Reagent (Roche). Small interfering RNA (siRNA) corresponding to coding sequence +136 to +156 of RbAp48 gene was synthesized by Hokkaido System Science: CGAGGAAUACAAAAUAUGGTT (sense), CCAUAUUUUGUAUUCCUCGTT (antisense). When the siRNA was transfected into HSG cells together with GFP plasmid, 73.4% of cells were found to be GFP+ HSG cells by flow cytometric analysis. Furthermore, the relative protein expression of RbAp48 to β-actin was reduced to ∼80% by the siRNA.
Real-time quantitative RT-PCR.
Total RNA was extracted from cultured HSG and MCF-7 cells using ISOGEN (Wako Pure Chemical), and reverse transcribed. Transcript levels of IRF-1, CIITA, and β-actin were performed using PTC-200 DNA Engine Cycler (Bio-Rad Laboratories) with SYBR Premix Ex Taq (Takara). Primer sequences were as follows: IRF-1, forward 5′-ACCCTGGCTAGAGATGCAGA-3′ and reverse 5′-CCTTTTCCCCTGCTTTGTATCG-3′; CIITA, forward 5′-CAGGCAGCAGAGGAGAAGTTCACCATC-3′ and reverse 5′-CCGTGAGGATCCGCACCAGTTTGGGG-3′; β-actin, forward 5′-AAATCTGGCACCACACCTTC-3′ and reverse 5′-GAGGCGTACAGGGATAGCA-3′.
Caspase activity.
Caspase activities were assayed using Caspase-Family Colorimetric Substrate Set (BioVision, Inc.). In brief, 100 μg cytoplasmic lysates from lacrimal glands, salivary glands, and spleen of WT and RbAp48-Tg mice were incubated with 200 μM Ac-YVAD-pNA (Caspase 1 substrate), at 37°C for 1 h. The absorbance of samples was read at 405 nm in a microplate reader.
Promoter assay.
For the measurement of the transcriptional activity of IRF-1, IRF-1 luciferase reporter vector (IRF-1/Luc) was purchased from Panomics. HSG cells plated in a 48-well plate were transiently transfected with 0.1 μg of IRF-1/Luc and 0.1 μg of pCMV-RbAp48 or mock plasmid and 0.05 μg of phRL-TK (Promega Corp.) as an internal control using the FuGENE6. The cells were incubated overnight and subsequently treated with IFN-γ. After 10 h, the cells were harvested and subjected to a luciferase assay by using a dual-luciferase reporter assay system (Promega Corp.) as per the manufacturer's instructions. Relative luciferase activity was expressed as the fold- increase relative to the activity of untreated controls after normalization to the relative background of Renilla luciferase activity.
Cell transfer.
CFSE-labeled splenic and cLN T cells (5 × 106) from WT and RbAp48-Tg mice were intravenously transferred into irradiated (700 cGy) C57BL/6.Ly5.1 mice. On the seventh day after the transfer, spleen cells were analyzed to measure homeostatic proliferation via CFSE dilution by flow cytometry. For induction of autoimmune lesions, total cells, T cells, or B cells from spleen cells (5 × 106) or cLN cells (5 × 106) from WT and RbAp48-Tg mice were intravenously transferred into Rag2−/− mice. At 6 wk after the transfer, the pathology of all the organs, including salivary and lacrimal glands, was analyzed. In addition, Rag2−/− hosts were ovariectomized (Ovx) or sham operated (Sham). Adoptive cell transfer was performed on the next day after Ovx or Sham.
In situ hybridization.
Mice were perfused transcardially with saline (0.9%) followed by 4% PFA. The salivary glands were collected and fixed in 4% PFA at 4°C for 3 h. 6 μm paraffin-embedded sections were prepared for ISH. The RNA probe (587 bp) of mouse IFN-γ was produced by RT-PCR using primers (T3, AATTAACCCTCACTAAAGGGACTGGCAAAAGGATGGTGAC; T7, TAATACGACTCACTATAGGGAGATACAACCCCGCAATCAC). Digoxigenin (DIG)-labeled antisense and sense control riboprobes were generated using DIG RNA labeling mix (Roche). The sections were pretreated with 10 μg/ml proteinase K for 10 min at room temperature and then hybridized with 1 μg/ml DIG-labeled probes at 45°C for 16 h. DIG was immunodetected with alkaliphosphatase-conjugated anti-DIG antibody. For positive controls, sections of spleen from lipopolysaccaride-injected mice were used. The probe was confirmed with the positive control sections, as shown in Fig. S6 B.
Human samples.
Immunostaining for RbAp48 and IL-18 or IFN-γ were performed using lip biopsy samples from human SS patients and controls. All samples were obtained from the Tokushima University Hospital, Tokushima, Japan. This study was approved by certification of the ethics board of Tokushima University Hospital. All subjects signed a written informed consent before enrollment. All patients with SS were female, had documented xerostomia and keratoconjunctivitis sicca, and fulfilled the criteria of the Ministry of Health, Labor, and Welfare of Japan for the diagnosis of SS. All patients with SS had focus scores of greater than two in their lip biopsy and all tested positive for autoantibodies against Ro. Analysis was performed under the certification of the ethics board of Tokushima University Hospital. Frozen sections were stained with anti–human RbAp48 mAb (BD) and Alexa Fluor 488 donkey anti–mouse IgG (H+L; Invitrogen) and Biotin-conjugated anti–human IL-18 (MBL) or IFN-γ (eBioscience) mAbs and Alexa Fluor 568–conjugated streptavidin and analyzed by confocal microscopy. The nuclear DNA was DAPI.
Statistics.
Student's t test was used for statistical analyses.
Online supplemental material.
Fig. S1 shows T cell phenotypes of thymus from RbAp48-Tg and WT mice. Fig. S2 shows T reg cells of thymus, spleen, and cLN from RbAp48-Tg and WT mice. Fig. S3 shows B1 cells in salivary glands and marginal B cells of spleen and cLN from RbAp48-Tg and WT mice. Fig. S4 shows the purified MSG cells, and images of control staining for the expressions of MHC class II, CD86, CD80, ICAM-1, IFN-γ, and IL18. Fig. S5 shows IRF-1 and CIITA mRNA of MCF-7 cells stimulated with Tam or transfected with pCMV-RbAp48. Fig. S6 shows IFN-γ concentration of tissue homogenates of lacrimal, salivary, and spleen from RbAp48-Tg and WT mice, and control sections for in situ hybridization of IFN-γ mRNA. Fig. S7 shows BAFF expression of salivary glands and spleen from RbAp48-Tg and WT mice. Fig. S8 shows the time courses of IL-18, IFN-γ, and HLA-DR expressions of HSG cells stimulated Tam or transfected with pCMV-RbAp48. Fig. S9 shows IFN-γ secretion from MCF-7 in response to IL-18. Fig. S10 shows control staining for RbAp48 expression together with IFN-γ or IL-18 in salivary glands from human SS patients and controls. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080174/DC1.
Supplementary Material
Acknowledgments
The authors thank Ai Nagaoka and Noriko Kino for their technical assistance; Kumio J. Tanaka, Mizue Yamanaka, and Shino Niki of OurGenic Co., Ltd. for analysis by in situ hybridization; and Prof. Noriaki Takeda for analysis of samples of human SS patients.
This work was supported in part by a Grant-in-Aid for Scientific Research (nos. 17109016, and 17689049) from the Ministry of Education, Science, Sport, and Culture of Japan, and from the Uehara Memorial Foundation.
The authors have no conflicting financial interests.
Abbreviations used: CIITA, class II transactivator; cLN, cervical LN; HSG, human salivary gland; IRF, IFN regulatory factor; MSG, mouse salivary gland; NOD, nonobese diabetic; RA, rheumatoid arthritis; RbAp48, retinoblastoma-associated protein 48; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome; Tg, transgenic.
N. Ishimura and R. Arakaki contributed equally to this paper.
References
- 1.Richardson, B. 2007. Primer: epigenetics of autoimmunity. Nat. Clin. Pract. Rheumatol. 3:521–527. [DOI] [PubMed] [Google Scholar]
- 2.Schoenborn, J.R., and C.B. Wilson. 2007. Regulation of interferon-g during innate and adaptive immune responses. Adv. Immunol. 96:41–101. [DOI] [PubMed] [Google Scholar]
- 3.Mathews, M.B., and R.M. Bernstein. 1983. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 304:177–179. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto, I., A. Staub, C. Benoist, and D. Mathis. 1999. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 286:1732–1735. [DOI] [PubMed] [Google Scholar]
- 5.Streilein, J.W., G.A. Wilbanks, and S.W. Cousins. 1992. Immunoregulatory mechanisms of the eye. J. Neuroimmunol. 39:185–200. [DOI] [PubMed] [Google Scholar]
- 6.Griffith, T.S., T. Brunner, S.M. Fletcher, D.R. Green, and T.A. Ferguson. 1995. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 270:1189–1192. [DOI] [PubMed] [Google Scholar]
- 7.Steinman, L. 1996. Multiple sclerosis. A coordinated immunological attack against myelin in the central nervous system. Cell. 85:299–302. [DOI] [PubMed] [Google Scholar]
- 8.Hutchings, P., L. O'Reilly, N.M. Parish, H. Waldmann, and A. Cooke. 1992. The use of a non-depleting anti-CD4 monoclonal antibody to re-establish tolerance to cells in NOD mice. Eur. J. Immunol. 22:1913–1918. [DOI] [PubMed] [Google Scholar]
- 9.Haskins, K., and M. McDuffie. 1990. Acceleration of diabetes in young NOD mice with a CD4+ islet-specific T cell clone. Science. 249:1433–1436. [DOI] [PubMed] [Google Scholar]
- 10.Nepom, G.T., and W.W. Kwok. 1998. Molecular basis for HLA-DQ associations with IDDM. Diabetes. 47:1177–1184. [DOI] [PubMed] [Google Scholar]
- 11.Stratmann, T., V. Apostolopoulos, V. Mallet-Designe, A.L. Corper, C.A. Scott, I.A. Wilson, A.S. Kang, and L. Teyton. 2000. The I-Ag7 MHC class II molecule linked to murine diabetes is a promiscuous peptide binder. J. Immunol. 165:3214–3225. [DOI] [PubMed] [Google Scholar]
- 12.Londei, M., J.R. Lamb, G.F. Bottazzo, and M. Feldmann. 1984. Epithelial cell expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 312:639–641. [DOI] [PubMed] [Google Scholar]
- 13.He, X.L., C. Radu, J. Sidney, A. Sette, E.S. Ward, and K.C. Garcia. 2002. Structural snapshot of aberrant antigen presentation linked to autoimmunity: the immunodominant epitope of MBP complexed with I-Au. Immunity. 17:83–94. [DOI] [PubMed] [Google Scholar]
- 14.Dallman, M.J., and D.W. Mason. 1983. Induction of Ia antigens on murine epidermal cells during the rejection of skin allografts. Transplantation. 36:222–224. [DOI] [PubMed] [Google Scholar]
- 15.Whitacre, C.C., S.C. Reingold, and P.A. O'Looney. 1999. A gender gap in autoimmunity. Science. 283:1277–1278. [DOI] [PubMed] [Google Scholar]
- 16.Whitacre, C.C. 2001. Sex differences in autoimmune disease. Nat. Immunol. 2:777–780. [DOI] [PubMed] [Google Scholar]
- 17.Apostolou, I., Z. Hao, K. Rajewsky, and H. von Boehmer. 2003. Effective destruction of Fas-deficient insulin-producing β cells in type 1 diabetes. J. Exp. Med. 198:1103–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamhamedi-Cherradi, S.E., S.J. Zheng, K.A. Maguschak, J. Peschon, and Y.H. Chen. 2003. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat. Immunol. 4:255–260. [DOI] [PubMed] [Google Scholar]
- 19.Rathmell, J.C., and C.B. Thompson. 2002. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 109:S97–S107. [DOI] [PubMed] [Google Scholar]
- 20.Stassi, G., and R. De Maria. 2002. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat. Rev. Immunol. 2:195–204. [DOI] [PubMed] [Google Scholar]
- 21.Ishimaru, N., K. Saegusa, K. Yanagi, N. Haneji, I. Saito, and Y. Hayashi. 1999. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjogren's syndrome through Fas-mediated apoptosis. Am. J. Pathol. 155:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishimaru, N., R. Arakaki, M. Watanabe, M. Kobayashi, M. Miyazaki, and Y. Hayashi. 2003. Development of autoimmune exocrinopathy resembling Sjogren's syndrome in estrogen deficient mice of healthy background. Am. J. Pathol. 163:1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishimaru, N., R. Arakaki, F. Omotehara, K. Yamada, K. Mishima, I. Saito, and Y. Hayashi. 2006. Novel role of RbAp48 for tissue-specific estrogen deficiency-dependent apoptosis in the exocrine glands. Mol. Cell. Biol. 26:2924–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saegusa, K., N. Ishimaru, K. Yanagi, K. Mishima, R. Arakaki, T. Suda, I. Saito, and Y. Hayashi. 2002. Prevention and induction of autoimmune exocrinopathy is dependent on pathogenic autoantigen cleavage in murine Sjögren's syndrome. J. Immunol. 169:1050–1057. [DOI] [PubMed] [Google Scholar]
- 25.Haneji, N., T. Nakamura, K. Takio, K. Yanagi, H. Higashiyama, I. Saito, S. Noji, H. Sugino, and Y. Hayashi. 1997. Identification of α-fodrin as a candidate autoantigen in primary Sjögren's syndrome. Science. 276:604–607. [DOI] [PubMed] [Google Scholar]
- 26.Berland, R., and H.H. Wortis. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20:253–300. [DOI] [PubMed] [Google Scholar]
- 27.Germain, R.N. 1994. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 76:287–299. [DOI] [PubMed] [Google Scholar]
- 28.Giroux, M., M. Schmidt, and A. Descoteaux. 2003. IFN-γ-induced MHC class II expression: transactivation of class II transactivator promoter IV by IFN regulatory factor-1 is regulated by protein kinase C-α. J. Immunol. 171:4187–4194. [DOI] [PubMed] [Google Scholar]
- 29.Hobart, M., V. Ramassar, N. Goes, J. Urmson, and P.F. Halloran. 1997. IFN regulatory factor-1 plays a central role in the regulation of the expression of class I and II MHC genes in vivo. J. Immunol. 158:4260–4269. [PubMed] [Google Scholar]
- 30.Ting, J.P., and J. Trowsdale. 2002. Genetic control of MHC class II expression. Cell. 109:S21–S33. [DOI] [PubMed] [Google Scholar]
- 31.Wright, K.L., and J.P. Ting. 2006. Epigenetic regulation of MHC-II and CIITA genes. Trends Immunol. 27:405–412. [DOI] [PubMed] [Google Scholar]
- 32.Elser, B., M. Lohoff, S. Kock, M. Giaisi, S. Kirchhoff, P.H. Krammer, and M. Li-Weber. 2002. IFN-γ represses IL-4 expression via IRF-1 and IRF-2. Immunity. 17:703–712. [DOI] [PubMed] [Google Scholar]
- 33.Shirasuna, K., M. Sato, and T. Miyazaki. 1981. A neoplastic epithelial duct cell line established form an irradiated human salivary gland. Cancer. 48:745–752. [DOI] [PubMed] [Google Scholar]
- 34.Howard, A.Y. 2006. Unravelling the pros and cons of interferon-γ gene regulation. Immunity. 24:506–507. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423–474. [DOI] [PubMed] [Google Scholar]
- 36.Bombardieri, M., F. Barone, V. Pittoni, C. Alessandri, P. Conigliaro, M.C. Blades, R. Priori, I.B. McInnes, G. Valesini, and C. Pitzalis. 2004. Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjogren's syndrome: relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate. Arthritis Res. Ther. 6:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muhl, H., and J. Pfeilschifer. 2004. Interleukin-18 bioactivity: a novel target for immunopharmacological anti-inflammatory intervention. Eur. J. Pharmacol. 500:63–71. [DOI] [PubMed] [Google Scholar]
- 38.Hurgin, V., D. Novick, and M. Rubinstein. 2002. The promoter of IL-18 binding protein: activation by an IFN-γ-induced complex of IFN regulatory factor 1 and CCAAT/enhancer binding protein-β. Proc. Natl. Acad. Sci. USA. 99:16957–16962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulukat, J., M. Bosmann, M. Nold, S. Garkisch, H. Kämpfer, S. Frank, J. Raedle, S. Zeuzem, J. Pfeilschifter, and H. Mühl. 2001. Expression and release of IL-18 binding protein in response to IFN-γ. J. Immunol. 167:7038–7043. [DOI] [PubMed] [Google Scholar]
- 40.Baccala, R., and A.N. Theofilopoulos. 2005. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 26:5–8. [DOI] [PubMed] [Google Scholar]
- 41.King, C., A. Ilic, K. Koelsch, and N. Sarvetnick. 2004. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 117:266–277. [DOI] [PubMed] [Google Scholar]
- 42.Carrasco-Marin, E., J. Shimizu, O. Kanagawa, and E.R. Unanue. 1996. The class II MHC I-Ag7 molecules from non-obese diabetic mice are poor peptide binders. J. Immunol. 156:450–458. [PubMed] [Google Scholar]
- 43.Rasooly, L., C.L. Burek, and N.R. Rose. 1996. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin. Immunol. Immunopathol. 81:287–292. [DOI] [PubMed] [Google Scholar]
- 44.Qian, Y.W., Y.C. Wang, R.E. Hollingsworth Jr., D. Jones, N. Ling, and E.Y. Lee. 1993. A retinoblastoma-binding protein related to a negative regulator of Ras in yeast. Nature. 364:648–652. [DOI] [PubMed] [Google Scholar]
- 45.Nicolas, E., S. Ait-Si-Ali, and D. Trouche. 2001. The histone deacetylase HDAC3 targets RbAp48 to the retinoblastoma protein. Nucleic Acids Res. 29:3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai, A., J.M. Lee, W.M. Yang, J.A. DeCaprio, W.G. Kaelin Jr., E. Seto, and P.E. Branton. 1999. RBP1 recruits both histone deacetylase-dependent and -independent repression activities to retinoblastoma family proteins. Mol. Cell. Biol. 19:6632–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicolas, E., V. Morales, L. Magnaghi-Jaulin, A. Harel-Bellan, H. Richard-Foy, and D. Trouche. 2000. RbAp48 belongs to the histone deacetylase complex that associates with the retinoblastoma protein. J. Biol. Chem. 275:9797–9804. [DOI] [PubMed] [Google Scholar]
- 48.Szende, B., I. Romics, and L. Vass. 1993. Apoptosis in prostate cancer after hormonal treatment. Lancet. 342:1422. [DOI] [PubMed] [Google Scholar]
- 49.Spyridopoulos, I., A. Sullivan, M. Kearney, J. Isner, and D. Losordo. 1997. Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis. Estradiol as a survival factor. Circulation. 95:1505–1514. [DOI] [PubMed] [Google Scholar]
- 50.Pelzer, T., M. Schumann, M. Neumann, T. deJager, M. Stimpel, E. Serfling, and L. Neyses. 2000. 17β-estradiol prevents programmed cell death in cardiac myocytes. Biochem. Biophys. Res. Commun. 268:192–200. [DOI] [PubMed] [Google Scholar]
- 51.Pike, C.J. 1999. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer's disease. J. Neurochem. 72:1552–1563. [DOI] [PubMed] [Google Scholar]
- 52.Bouker, K.B., T.C. Skaar, D.R. Fernandez, K.A. O'Brien, R.B. Riggins, D. Cao, and R. Clarke. 2004. Interferon regulatory factor-1 mediates the proapoptotic but not cell cycle arrest effects of the steroidal antiestrogen ICI 182,780 (faslodex, fulvestrant). Cancer Res. 64:4030–4039. [DOI] [PubMed] [Google Scholar]
- 53.Davidson, A., and B. Diamond. 2001. Autoimmune diseases. N. Engl. J. Med. 345:340–350. [DOI] [PubMed] [Google Scholar]
- 54.Marrack, P., J. Kappler, and B.L. Kotzin. 2001. Autoimmune disease: why and where it occurs. Nat. Med. 7:899–905. [DOI] [PubMed] [Google Scholar]
- 55.Cenci, S., G. Toraldo, M.N. Weitzmann, C. Roggia, Y. Gao, and W.P. Qian. 2003. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc. Natl. Acad. Sci. USA. 100:10405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodgin, J.B., and N. Maeda. 2002. Minireview: estrogen and mouse models of atherosclerosis. Endocrinology. 143:4495–4501. [DOI] [PubMed] [Google Scholar]
- 57.Mikkelsen, T.R., J. Brandt, H.J. Larsen, B.B. Larsen, K. Poulsen, J. Ingerslev, N. Din, and J.P. Hjorth. 1992. Tissue-specific expression in the salivary glands of transgenic mice. Nucleic Acids Res. 20:2249–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White, S.C., and G.W. Casarett. 1974. Induction of experimental autoallergic sialadenitis. J. Immunol. 122:178–185. [PubMed] [Google Scholar]
- 59.Saegusa, K., N. Ishimaru, K. Yanagi, R. Arakaki, K. Ogawa, I. Saito, N. Katunuma, and Y. Hayashi. 2002. Cathepsin S-inhibitor prevents autoantigen presentation and autoimmunity. J. Clin. Invest. 110:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.