Abstract
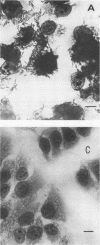
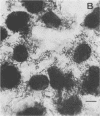
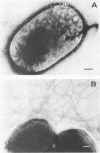
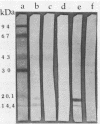
Two Escherichia coli strains (B/M 369 and C-35) belonging to enteropathogenic serogroup O86 were isolated from patients with infantile diarrhea and studied with respect to their cellular adherence properties. Both strains exhibited adherence (Ad+) to HEp-2 and HeLa cell monolayers in vitro and expressed mannose-resistant hemagglutinating (MRHA+) activity towards human, chicken, and sheep (but not mouse, rabbit, or guinea pig) erythrocytes. Cellular adherence properties of both strains could be substantially reduced by pronase treatment and by heat treatment (100 degrees C for 5 min) of bacteria. Electron microscopic examination failed to reveal fimbria- or pilus-like structures on the bacterial cell surface. Conjugation experiments conducted with these strains suggested that both MRHA and HEp-2 and HeLa cell adherence factors were encoded by the same plasmid, with a size of 55 to 57 megadaltons (MDa). Further biochemical studies indicated that the cellular adherence factors were associated with cell surface structures of bacteria that were proteinaceous in nature. An antiserum, rendered specific for the 57-MDa plasmid (pRP201) products of B/M 369 by adsorption, reacted with both MRHA+ Ad+ strains, B/M 369 and C-35, but not with their 57- or 55-MDa plasmidless MRHA- Ad- transconjugants or with other MRHA- Ad- E. coli strains. Immunological studies showed that the absorbed antiserum recognized two proteins with subunit molecular sizes of 18 and 14.5 kDa that were present on the cell surfaces of both strains. Furthermore, the absorbed antiserum at subagglutinating dilutions did inhibit, although only partially, the MRHA and HEp-2 and HeLa cell adherence activities of both E. coli strains. All these results would indicate that some of the E. coli strains belonging to enteropathogenic serogroups express their adherence potential through factors that were hitherto unrecognized.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Scotland S. M., Willshaw G. A., Rowe B. HEp-2 adhesion and the expression of a 94 kDa outer-membrane protein by strains of Escherichia coli belonging to enteropathogenic serogroups. J Gen Microbiol. 1988 May;134(5):1315–1321. doi: 10.1099/00221287-134-5-1315. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Clegg S., Pauley J. A. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):738–748. doi: 10.1128/iai.25.2.738-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhar J., Perry R., Golecki J. R., Hoschutzky H., Jann B., Jann K. Nonfimbrial, mannose-resistant adhesins from uropathogenic Escherichia coli O83:K1:H4 and O14:K?:H11. Infect Immun. 1987 Aug;55(8):1837–1842. doi: 10.1128/iai.55.8.1837-1842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Taga S., Takeda Y., Miwatani T. Modified Elek test for detection of heat-labile enterotoxin of enterotoxigenic Escherichia coli. J Clin Microbiol. 1981 Jan;13(1):1–5. doi: 10.1128/jcm.13.1.1-5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge M. S., Palchaudhuri S. Use of a known plasmid and transposon as tracers for the incompatibility classification of a cryptic plasmid. Mol Gen Genet. 1982;186(2):282–288. doi: 10.1007/BF00331863. [DOI] [PubMed] [Google Scholar]
- Kabir S. Characterization of the lipopolysaccharide from Vibrio cholerae 395 (Ogawa). Infect Immun. 1982 Dec;38(3):1263–1272. doi: 10.1128/iai.38.3.1263-1272.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldini M. M., Kaper J. B., McNeish A. S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987 Jan;55(1):78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laporta M. Z., Silva M. L., Scaletsky I. C., Trabulsi L. R. Plasmids coding for drug resistance and localized adherence to HeLa cells in enteropathogenic Escherichia coli O55:H- and O55:H6. Infect Immun. 1986 Feb;51(2):715–717. doi: 10.1128/iai.51.2.715-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Scaletsky I. C., Kaper J. B., Levine M. M., Trabulsi L. R. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985 May;48(2):378–383. doi: 10.1128/iai.48.2.378-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Birch-Andersen A., Duguid J. P., Stenderup J., Orskov F. An adhesive protein capsule of Escherichia coli. Infect Immun. 1985 Jan;47(1):191–200. doi: 10.1128/iai.47.1.191-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Libaek L. B., Schoolnik G. K. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect Immun. 1987 Sep;55(9):2052–2056. doi: 10.1128/iai.55.9.2052-2056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Milani S. R., Trabulsi L. R., Travassos L. R. Isolation and characterization of the localized adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1988 Nov;56(11):2979–2983. doi: 10.1128/iai.56.11.2979-2983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavendale A., Old D. C. Haemagglutinins and adhesion of Escherichia coli to HEp2 epithelial cells. J Med Microbiol. 1985 Dec;20(3):345–353. doi: 10.1099/00222615-20-3-345. [DOI] [PubMed] [Google Scholar]
- Templeton W. C., 3rd, Wawrukiewicz A., Melo J. C., Schiller M. G., Raff M. J. Anaerobic osteomyelitis of long bones. Rev Infect Dis. 1983 Jul-Aug;5(4):692–712. doi: 10.1093/clinids/5.4.692. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Williams P. H., Knutton S., Brown M. G., Candy D. C., McNeish A. S. Characterization of nonfimbrial mannose-resistant protein hemagglutinins of two Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):592–598. doi: 10.1128/iai.44.3.592-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., Kuipers O., Tommassen J., Lugtenberg B. O-antigenic chains of lipopolysaccharide prevent binding of antibody molecules to an outer membrane pore protein in Enterobacteriaceae. Microb Pathog. 1986 Feb;1(1):43–49. doi: 10.1016/0882-4010(86)90030-6. [DOI] [PubMed] [Google Scholar]