Abstract
Inactivated Bacillus anthracis spores given with protective antigen (PA) contribute to immunity against anthrax in several animal models. Antiserum raised against whole irradiated B. anthracis spores has been shown to have anti-germination and opsonic activities in vitro. Based on these observations, we hypothesized that surface-exposed spore proteins might serve as supplemental components of a PA-based anthrax vaccine. The protective anti-spore serum was tested for reactivity with recombinant forms of 30 proteins known, or believed to be, present within the B. anthracis exosporium. Eleven of those proteins were reactive with this antiserum, and, subsequently a subset of this group was used to generate rabbit polyclonal antibodies. These sera were evaluated for recognition of the immunogens on intact spores generated from Sterne strain, as well as from an isogenic mutant lacking the spore surface protein Bacillus collagen-like antigen (BclA). The data were consistent with the notion that the antigens in question were located beneath BclA on the basal surface of the exosporium. A/J mice immunized with either the here-to-for hypothetical protein p5303 or the structural protein BxpB, each in combination with subprotective levels of PA, showed enhanced protection against subcutaneous spore challenge. While neither anti-BxpB or anti-p5303 antibodies reduced the rate of spore germination in vitro, both caused increased uptake and lead to a higher rate of destruction by phagocytic cells. We conclude that by facilitating more efficient phagocytic clearance of spores, antibodies against individual exosporium components can contribute to protection against B. anthracis infection.
Keywords: Bacillus anthracis, Spore, Exosporium, Vaccine
1. Introduction
Bacillus anthracis, the etiological agent of anthrax, is a spore-forming, Gram-positive bacterium. B. anthracis spores, the highly resistant infectious stage of the organism capable of remaining dormant yet viable for decades, form in response to stressful environmental conditions such as desiccation and inadequate supplies of nutrients [1]. Spores can establish infection in a host through the cutaneous, oral, or inhalational routes, each of which provide permissive environments for spores to germinate, grow into bacilli, and elaborate the toxins that ultimately kill the host [2]. Although herbivores are the primary target of anthrax spores within the environment and natural human infection is incidental, the potential for the infection of large populations by intentional distribution of spores was highlighted by the biological attack conducted through the U.S. mail system in 2001 [3].
While post-exposure antibiotic therapy is an effective treatment for rapidly diagnosed anthrax infection [4], prophylactic immunization offers the possibility of protection to potentially vulnerable populations prior to exposure. The currently licensed vaccine is the anthrax vaccine adsorbed (AVA), a preparation consisting of a formalin-treated, aluminum salt-adsorbed, cell-free culture filtrate from a non-encapsulated attenuated strain of B. anthracis [5]. The protection afforded by AVA is primarily attributable to antibodies raised against protective antigen (PA) [6], the cell-binding component of both edema toxin and lethal toxin and an essential element of the deadly toxemia mediated by anthrax infection [7]. Indeed, correlates of protection for anthrax immunizations are based on anti-PA antibody titers [8–13], and as a result, PA is an essential component in any potential future anthrax vaccine candidate. However, though considered safe and effective [14], AVA is plagued by issues related to reactogenicity, availability, lot-to-lot PA dosage variability, a multi-dose vaccine regimen, and adverse public perception toward the anthrax vaccine [5,15]. Furthermore, while defined recombinant PA (rPA) sources [16], novel delivery platforms [17,18], and more effective adjuvants [19] offer the possibility of improved immune responses to PA, immunization studies in a variety of animals models cite the variability of the protection conferred by vaccines based solely on PA [20–24].
An alternative approach to anthrax vaccinology might be to target not only the toxin produced by the vegetative bacillus but also the spore that is the essential component for establishing infection. Multiple studies demonstrate the capacity of attenuated live spore vaccines to confer protection against anthrax spore challenge [24–28]. Live-spore vaccines appear to be more protective than PA-based vaccines against challenge with virulent strains of B. anthracis [24,27,29], perhaps because of the broader immune response these spore vaccines likely generate. Brossier et al. [30] demonstrated that the addition of formaldehyde-inactivated spores to a PA-based vaccine conferred greater protection against spore challenge than PA alone, despite both formulations eliciting similar levels of toxin-neutralizing activity. While these studies in aggregate strongly indicate that responses to spore-associated antigens contribute to protective immunity, the fact that whole spore-based vaccines are unacceptable for human use in the United States due to safety concerns requires the identification of individual spore antigens that might be added to the current PA-based vaccine to recapitulate the benefits of whole spores and create a more efficacious vaccine formulation.
To identify the best spore antigen candidates, we chose to focus on proteins located on the outermost surface of the spore. Unlike most Bacillus species, B. anthracis spores are covered with an exosporium, a “balloon-like” structure that loosely envelops the outer surface of the spore coat and consists of a lattice-work basal layer and a covering of hair-like projections [31]. The hair-like appendages are constructed from the immunodominant spore glycoprotein BclA [32,33] and represent a tantalizing target for vaccine efforts. Recent studies demonstrated that mice challenged with B. anthracis spores were better protected following immunization with PA plus BclA, administered either in recombinant form [34] or on a BclA-encoding plasmid [35], as compared to immunization with PA alone. Given this proof of concept for targeting spore antigens as effective immunogens, in this investigation we sought to screen known components of the exosporium and spore coat for additional potential vaccine candidates. From a subset of spore antigens that we verified to be present in the outer structures of the spore, and to be targets of the immune response generated against whole spores, we identified a pair of proteins capable of enhancing protection against lethal anthrax spore challenge in A/J mice following immunization with suboptimal amounts of rPA. Unlike antiserum generated against whole spores, antibodies directed against individual spore components were not capable of significantly reducing spore germination. However, a possible explanation for the protective efficacy of these spore antigens might be found in the capacity of the antibodies to enhance spore uptake and killing by professional phagocytes.
2. Materials and methods
2.1. Bacterial strains
B. anthracis Sterne strain (toxigenic, unencapsulated, Naval Medical Research Center, Silver Spring, MD) and an isogenic deletion mutant lacking the Bacillus collagen-like antigen (BclA) gene (ΔbclA) [36] were used for in vitro and in vivo experiments.
2.2. Preparation of B. anthracis spores
Spores were produced as previously described [37]. Briefly, single colonies of B. anthracis Sterne strain or ΔbclA were taken from brain heart infusion (BHI) agar plates and inoculated into BHI broth for culture overnight at 37 °C. Each culture was spread onto modified germination (G) medium [38] agar plates (0.2% yeast extract, 0.2% (NH4)2SO4, 1.5% Bacto agar, 0.0025% CaCl2 dihydrate, 0.05% K2HPO4, 0.02% MgSO4 heptahydrate, 0.005% MnSO4 quatrahydrate, 0.0005% ZnSO4 dihydrate, 0.0005% CuSO4 pentahydrate, 0.00005% FeSO4 heptahydrate). The plates were incubated at 30 °C for 10–14 days in the dark. Colonies scraped from the surface of the agar were re-suspended in distilled water, washed twice in distilled water, and heat-treated at 65 °C for 1 h to kill any viable vegetative cells. Purification of spores was done with 58% (v/v) Renografin (Renocal-76, Bracco Diagnostics, Princeton, NJ, USA) diluted in dH2O. Spores were layered onto the 58% Renografin solution and centrifuged at 6000 × g for 60 min in a swinging bucket rotor. The sedimented spores were washed twice with distilled water. After the final sedimentation, the spores were re-suspended in distilled water to yield a final concentration of 109–1010 colony-forming units (cfu)/ml, as determined by vegetative outgrowth on BHI plates.
2.3. Preparation of spore surface protein extract (SSPE)
Analysis of the proteins contained within the exosporium was achieved by removal of the exosporium from whole spores via chemical extraction according to a method previously described [39]. Briefly, 0.5 ml of heat-activated spores (109–1010 cfu/ml) were pelleted at 7000 × g for 15 min at 4 °C. Spores were washed twice with water and resuspended in 0.5 ml of extraction buffer that contained 0.1 M DTT, 0.1 M NaCl, 0.5% SDS, pH 10. Samples were incubated in a 37 °C shaking water bath for 2.5 h and pelleted, and the resulting supernatant was collected. Supernatants were then filtered through a 0.2 μm Corning filter and dialyzed against PBS.
2.4. Preparation of recombinant proteins
Our procedures for the construction of Escherichia coli strains that expressed recombinant proteins with an N-terminal six-histidine tag, and for purification of those proteins by nickel affinity chromatography are described in detail elsewhere [36]. Briefly, genomic DNA was extracted from B. anthracis strains with the Easy-DNA kit (Invitrogen, Carlsbad, CA, USA). NdeI–BamHI, XhoI–BamHI or XhoI–BglII fragments that contained each gene of interest were amplified from the Sterne genome by polymerase chain reactions (PCR) using the Expand High Fidelity PCR system (Roche Diagnostics, Indianapolis, IN, USA) in a PTC200 Peltier Thermal Cycler (MJ Research, Bio-Rad, Hercules, CA, USA). Primer sets designed from the flanking sequences of the targeted gene [National Center for Biotechnology Information (NCBI) at website http://www.ncbi.nlm.nih.gov.lrc1.usuhs.edu/entrez/viewer.fcgi?db=nucleotide&val=AE017225] are listed in Supplemental Materials, Table 1. PCR products were purified with the QIAEX II Gel Extraction Kit (Qiagen, Valencia, CA 91355, USA), and the DNA fragments were ligated into the expression vector pET15b (Novagen, San Diego, CA). The resulting expression plasmids are listed in Supplemental Materials, Table 2. DNA sequences were verified with the ABI Prism Big Dye method (Applied Biosystems, Forest City, CA, USA) by the Biomedical Instrumentation Center at the Uniformed Services University, then recombinant plasmids were transformed into E. coli BL21(DE3) pLysS according to the pET system manual (Novagen). His-tagged proteins were expressed from transformants and subjected to His-Trap nickel affinity column chromatography with the AKTA fast protein liquid chromatography system (GE Healthcare, Piscataway, NJ).
Table 1.
B. anthracis genes cloned and expressed in this study
| Sterne locus | Ames locus | Function | ORF (bp) | Protein size (kDa) | Anti-spore reactivity (31101-01) | Reference |
|---|---|---|---|---|---|---|
| BAS0108 | BA0108 | Translation elongation factor Tu | 1188 | 42.9 | − | [43,44] |
| BAS0238 | BA0252 | Alanine racemase | 1170 | 43.6 | − | [32,43–45] |
| BAS0253 | BA0267 | GroEL | 1635 | 57.4 | − | [43,46] |
| BAS0340 | BA0355 | CotB homolog | 522 | 19.4 | − | [43] |
| BAS0766 | BA0803 | CotJC | 570 | 21.6 | − | [44,47] |
| BAS0767 | BA0804 | CotJB | 276 | 10.9 | − | [44] |
| BAS0768 | BA0805 | CotJA | 216 | 8.4 | − | [44] |
| BAS1130 | BA1222 | BclA | 1203 | 36.8 | + | [32,33] |
| BAS1141 | BA1234 | CotZ1 | 459 | 16.1 | + | [43,44] |
| BAS1144 | BA1237 | BxpB | 504 | 17.3 | + | [32,43,44] |
| BAS1145 | BA1238 | CotZ2 | 471 | 16.8 | − | [43,44] |
| BAS1378 | BA1489 | Fe–Mn superoxide dismutase (SOD15) | 915 | 36.1 | + | [32,44,45] |
| Δ1–96 BAS1378 | Δ1–96 BA1489 | Truncated Fe–Mn superoxide dismutase (SOD15) | 627 | 24.6 | + | [32,45] |
| BAS1655 | BA1786 | ExsE | 957 | 35.8 | − | [48] |
| BAS2008 | BA2162 | BxpA | 705 | 27.7 | − | [32,44] |
| BAS2138 | BA2292 | Hypothetical protein (p2138) | 756 | 28.5 | − | [44] |
| BA2150 homolog | BA2150 | ExsG | 153 | 5.4 | − | [48] |
| BAS2174 | BA2332 | BxpC | 399 | 14.4 | − | [43] |
| BAS2377 | BA2554 | Hypothetical protein (p2377) | 330 | 12 | + | [43,44] |
| BAS2439 | BA2617 | ExsD | 465 | 17.4 | + | [48] |
| BAS2693 | BA2888 | Inosine-uridine preferring nucleoside hydrolase | 969 | 36.3 | − | [47] |
| BAS2986 | BA3211 | Hypothetical protein (p2986) | 474 | 17.8 | − | [47] |
| BAS3402 | BA3668 | Glycosyl hydrolase, family 18 | 1293 | 48.1 | − | [44] |
| BAS3619 | BA3906 | CotE | 543 | 20.4 | − | [48] |
| BAS3957 | BA4266 | Hypothetical protein (p3957) | 363 | 13.4 | + | [44] |
| BAS4177 | BA4499 | Mn superoxide dismutase (SODA1) | 612 | 22.6 | + | [44] |
| BAS4383 | BA4722 | ThiJ/PfpI family protein | 663 | 24 | + | [44] |
| BAS4544 | BA4898 | Small, acid-soluble spore protein B | 198 | 6.8 | − | [44] |
| BAS5241 | BA5640 | Cell wall hydrolase | 423 | 16.1 | − | [44] |
| BAS5242 | BA5641 | YwdL | 438 | 16.2 | + | [44] |
| BAS5303 | BA5699 | Hypothetical protein (p5303) | 402 | 15 | + | [44] |
Table 2.
Survival, mean time-to-death (MTD), and serological response of mice vaccinated simultaneously with rPA plus recombinant spore protein and challenged s.c.
| Group | Immunization
|
Survivala | MTD | Mean anti-PA response (OD450)b | Mean anti-spore protein response (OD450)c | |
|---|---|---|---|---|---|---|
| Day 1 | Day 15 | |||||
| 1 | PBS | PBS | 0/5 | 4.2 | 0.07 | N/A |
| 2 | PBS | PA (50 ng) | 2/5 | 8.0 | 1.29* | N/A |
| 3 | CotZ1 (10 μg) | PA (50 ng) + CotZ1 (10 μg) | 0/5 | 4.5 | 0.31 | 1.12 |
| 4 | BxpB (10 μg) | PA (50 ng) + BxpB (10 μg) | 0/5 | 5.3 | 0.11 | 2.65 |
| 5 | SOD15 (10 μg) | PA (50 ng) + SOD15 (10 μg) | 0/5 | 5.2 | 0.15 | 2.88 |
| 6 | p2366 (10 μg) | PA (50 ng) + p2366 (10 μg) | 0/5 | 4.6 | 0.24 | 0.18 |
| 7 | p3957 (10 μg) | PA (50 ng) + p3957 (10 μg) | 0/5 | 4.0 | 0.13 | 0.58 |
| 8 | SODA1 (10 μg) | PA (50 ng) + SODA1 (10 μg) | 0/5 | 4.8 | 0.30 | 3.10 |
| 9 | YwdL (10 μg) | PA (50 ng) + YwdL (10 μg) | 0/5 | 4.6 | 0.20 | 3.05 |
| 10 | p5303 (10 μg) | PA (50 ng) + p5303 (10 μg) | 0/5 | 5.8 | 0.19 | 2.94 |
Mice were challenged on Day 28 with 10 LD50s Sterne spores. The 50% lethal dose of Sterne strain is approximately 1.13 × 103 spores.
Optical density measurement at 450 nm for a 1:300 antiserum dilution. OD450 for positive control (monoclonal anti-PA antibody) was 1.68. OD450 for negative control (NRS) was 0.04.
Optical density measurement at 450 nm for a 1:200 antiserum dilution. OD450 for positive controls (polyclonal anti-spore protein IgGs) ranged from 3.23 to 3.43. OD450 for negative control (NRS) was 0.04.
P ≤ 0.002 compared to all other groups.
2.5. Immune sera and antibody preparation
Polyclonal antibodies were generated against each recombinant protein by methods previously described [36]. Briefly, rabbits were vaccinated monthly with 50 μg of purified recombinant protein in Freund’s complete adjuvant for the first inoculation and Freund’s incomplete adjuvant for all subsequent immunizations. Production bleeds were taken and analyzed for specific antibodies beginning at month 5, with final bleeds taken at month 12. Immunoglobulins G were purified from each final serum sample by passage over a protein G column (Pierce Biotechnology, Rockford, IL). IgG preparations were then affinity-purified on an UltraLink® Biosupport column (Pierce Biotechnology) conjugated to 5 mg of recombinant target protein. Resulting antibody concentrations were determined by a microtiter BCA assay (Pierce Biotechnology).
2.6. Western blot analysis
2.6.1. Screening of recombinant protein candidates
Purified recombinant forms of spore proteins were screened as follows. Purified His-tagged proteins (100 ng/lane) were separated by electrophoresis on a 4–20% Tris-glycine sodium dodecyl sulfate (SDS) polyacrylamide gel (Invitrogen), transferred onto Optitran BA-S 83 reinforced nitrocellulose (Whatman GmbH, Dassel, GE), and blocked overnight at 4 °C in PBST (0.5% Tween-20) with 5% nonfat dry milk (PBSTM). To verify the presence of the recombinant protein, blots were then incubated for 1 h at room temperature with a His-Tag® monoclonal antibody (Qiagen, Madison, WI) diluted 1:4000 in PBSTM, washed three times for 5 min in PBST, incubated for 1 h at room temperature with a secondary antibody [goat anti-mouse IgG conjugated to horseradish peroxidase (Bio-Rad)] diluted 1:16,000 in PBST, washed three times for 5 min each in PBST, and developed with Lumigen PS-3 Acridan (GE Healthcare UK, Buchinghamshire, UK) and Kodak BioMax XAR film (Kodak, Rochester, NY). To verify recognition of the recombinant protein by antiserum directed against whole spores, blots were incubated for 1 h at room temperature with affinity-purified anti-spore rabbit polyclonal serum (designated 311001-01 and kindly provided by Naval Medical Research Center, protein G purified, original concentration 4.2 mg/ml) diluted to 0.1 μg/ml in PBSTM. The blot was subsequently washed three times for 5 min each in PBST, incubated for 1 h at room temperature with a secondary antibody [goat anti-rabbit IgG conjugated to horseradish peroxidase (Bio-Rad)] diluted 1:16,000 in PBST, washed three times for 5 min in PBST, and developed as described above.
2.6.2. Screening of SSPE
Antigens within SSPE were separated by electrophoresis on 4–20% Tris-glycine SDS-PAGE gels loaded with 5 μl of SSPE diluted to a final volume of 20 μl in distilled water and transferred to nitrocellulose. The nitrocellulose was blocked with PBSTM and incubated with affinity-purified polyclonal anti-spore protein IgG standardized at 1 mg/ml and diluted 1:5000 in PBSTM for 1 h. Following three, 5 min washes at room temperature with PBST, the nitrocellulose was probed with goat anti-rabbit IgG conjugated to horseradish peroxidase and developed as above.
2.7. Immunoelectron microscopy
Bacterial spores were fixed in 4% formaldehyde (freshly prepared from paraformaldehyde crystals) in PBS (pH 7.0) for a minimum of 8 h. Following three 10-min washes in PBS, spores were labeled by an immunogold technique with either pre- or post-embedding methods. For pre-embedding immunolabeling, spores were suspended in blocking buffer [PBS supplemented with 1 mg/ml BSA Fraction V (EMD Chemicals Inc., Gibbstown, NJ), 2% normal rabbit serum and 0.5% cold water fish skin gelatin (Electron Microscopy Sciences, Hatfield, PA)] for 45 min. Spores were then incubated with primary antibody (affinity-purified anti-spore protein polyclonal IgG) for 1 h followed by secondary for 1 h. The secondary antibody consisted of goat anti-rabbit IgG conjugated to 10 nm gold particles (Electron Microscopy Sciences). The spores were then incubated for 10 min in 4% aqueous electron microscopy grade glutaraldehyde (Tousimis, Rockville, MD) followed by 10 min in 50 mM glycine to quench unreacted aldehyde groups. Three 10 min washes in PBS were done between each step in the labeling procedure. Spores were dehydrated in a graduated series of ethanol and infiltrated with Spurr’s epoxy resin (Electron Microscopy Sciences). Thin sections of samples were then prepared and collected on 200 mesh copper grids. For post-embedding labeling, spores were dehydrated in a graduated series of ethanol and then infiltrated in LR White acrylic resin (London Resin Company Limited, Berkshire, England) at room temperature. Following overnight polymerization of the resin at −20 °C in a UV Cryo Chamber (Electron Microscopy Sciences), thin sections were collected on 200 mesh nickel grids. Grids that contained sections were then floated on a drop of blocking buffer for 30 min, and the grids were then incubated in primary followed by secondary antibodies (see above) for 1 h each. Three 10-min washes in PBS were done between each step in the labeling procedure. All grids that contained samples were then stained for 10 min in 2% aqueous uranyl acetate and for 5 min in Reynold’s lead citrate. Samples were examined on a Philips CM100 electron microscope (FEI Company, Hillsboro, OR), and images were collected with a Spot Insight 4MP digital camera (Diagnostic Instruments, Sterling Heights, MI).
2.8. Mouse immunization and challenge protocol
Immunization experiments were done as previously described [34], with modifications. Briefly, 6–8-week-old female A/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and quarantined for 1 week before use. Preimmune serum samples were collected from mice by tail nicks. Two immunization protocols were used. In all cases, proteins were suspended in PBS (pH 7.4) and mixed 1:1 by volume with TiterMax Gold (TiterMax USA Inc., Nor-cross, GA) for a total volume of 100 μl. In protocol #1, the mice were immunized intraperitoneally (i.p.) on Day 1 with 10 μg of recombinant spore protein (BxpB, YwdL, SOD15, SODA1 or p5303). Control mice received PBS plus TiterMax Gold mixed 1:1 by volume. On Day 15, mice received a boost containing 10 μg of recombinant spore protein plus 50 ng of rPA. Control mice received either 50 ng of rPA (“PA Only” Control) or simply PBS with TiterMax Gold (“PBS Only” Control). In protocol #2, the mice were immunized i.p. on Day 1 with 50 ng of purified rPA, followed on Day 15 with 10 μg of recombinant spore protein. Following each protocol, mice were bled by tail nick on Day 28 and challenged on Day 29 with 10 times the 50% lethal dose (LD50) of Sterne spores (about 104 spores) injected subcutaneously (s.c.) behind the right foreleg. The animals were then monitored for survival twice daily for 14 days after challenge.
2.9. ELISA analysis
2.9.1. Anti-spore ELISA
Analysis of antigen target accessibility on the spore surface was done by enzyme-linked immunosorbent assays (ELISA) with affinity-purified anti-spore protein polyclonal IgG directed against whole spores as a probe. Briefly, 96-well plates were coated with 0.1 ml of a 1 × 108 cfu/ml solution of Sterne or ΔbclA spores and incubated overnight at 4 °C. Spores were removed and wells blocked overnight at 4 °C in PBST with 3% BSA and 0.05% Tween-20 (PBSTB). After removal of the PBSTB from the wells, antibodies standardized at 1 mg/ml were added to wells at an initial dilution of 1:50 [in PBST (0.05% Tween-20)] and serially diluted 1:2 in PBST to a final dilution of 1:51,200. After a 1-h incubation at room temperature, wells were washed four times with PBST, and a secondary antibody (goat anti-rabbit IgG conjugated to horseradish peroxidase) was added at a dilution of 1:5000 in PBST. After a 1-h incubation at room temperature, wells were washed four times with PBST. The secondary antibody was detected with 3,3,5,5-tetramethylbenzidine peroxidase (Bio-Rad), and the microtiter plates were incubated at room temperature for 15 min. An aliquot (100 μl) of 1 M H2SO4 was then added to each well to quench the reaction, and the color intensity in each well was assessed by measuring the optical density at 450 nm (OD450) with an ELISA microtiter reader (Microtek; Molecular Devices, Sunnyvale, CA).
2.9.2. ELISAs to measure mouse anti-rPA and anti-spore protein antibodies
ELISAs were done to assess anti-rPA and anti-recombinant protein mouse sera IgG responses by methods described above, with modifications. Briefly, purified protein (100 ng of each in 100 μl PBS) was used to coat the wells of a “U”-bottom 96-well microtiter plate (Thermo Electron Corp., Milford, MA), and the microtiter plates were incubated at 4 °C overnight. Wells were blocked and washed as described above before 100 μl of a 1:100 dilution of either pre- or post-immunization serum sample was added to the first well of the plate and serially diluted 1:2 out to 1:12,800. The microtiter plates were incubated for 2 h at 37 °C, after which the wells of the plates were washed three times with PBST. Next, 100-μl aliquots of the secondary antibody (goat anti-mouse IgG conjugated to horseradish peroxidase) diluted 1:10,000 in PBS were added to wells, and plates were incubated at room temperature for 1 h. Each mouse serum was assessed in triplicate. Samples were developed and measured by techniques described above. The average intensity of the ELISA reading from the post-immune serum samples was determined after subtracting the average OD450 readings of the preimmune serum from the same animal. The positive controls for the anti-rPA, anti-BxpB and anti-p5303 protein ELISAs were a monoclonal anti-PA antibody (14B7, originally prepared by S.F. Little and provided by the National Naval Medical Center [40]) and affinity-purified anti-BxpB and anti-p5303 polyclonal IgGs standardized to 1 mg/ml in PBS. The negative control was protein-G purified normal rabbit sera (NRS) that was kindly provided by Dr. Jill Czarnecki, National Naval Medical Center.
2.10. Germination assay
To examine the possibility of germination inhibition by polyclonal antibodies directed against spore proteins, 1 × 108 heat-activated spores were incubated at 37 °C for 30 min with 500 μg of anti-spore protein IgG prior to the measurement of germination rate. Anti-spore serum 311001-01 was used as a positive control, while protein-G purified NRS was used as a negative control. Germination efficiencies of the strains were compared by culturing spores in 5% BHI broth at 37 °C and 225 rpm, taking samples from the broth cultures at various times, and heat-inactivating the vegetative cells at 68 °C for 1 h, as previously described [36]. The heat-treated samples were then sub-cultured onto trypticase soy agar (TSA) plates to enumerate the residual heat-resistant spores. Within each experiment, three samples of each strain were heat-treated and counted, and the geometric mean cfu for like samples was determined. The heat-resistant cfu over time was determined for each strain in three independent experiments.
2.11. Macrophage infection model
The interaction of spores with macrophages of the RAW264.7 cell line (American Type Culture Collection, ATCC, Rockville, MD, USA) was studied by methods detailed earlier [41,42], with modifications. Briefly, samples of 1 × 108 heat-activated spores were pre-incubated for 1 h at 37 °C with 50 μg of anti-spore protein IgG. Anti-spore serum 311001-01 was used as a positive control, while protein-G purified NRS was used as a negative control. After 1 h, spores were diluted in serum-free DMEM to a final concentration of 5 × 106 spores/ml. The cell line was grown in Dulbecco’s Minimal Essential Medium (DMEM) with 10% fetal bovine serum (FBS, Lonza, Walkersville, MD, USA). Seed cultures were subcultured onto six-well plates and incubated at 37 °C in air with 5% CO2 for 3–4 days, until cells reached (0.8–1.2) × 106 cells/well. Cells were washed three times with PBS and infected with ~5 cfu per cell. Cells were incubated at 37 °C in air with 5% CO2 for 45 min. Unphagocytosed spores were then removed by washing the wells three times with PBS. Serum-free DMEM that contained 50 μg/ml gentamicin was added, and cells were incubated at 37 °C in air with 5% CO2 for 45 min to kill extracellular bacteria. The cells were washed three times with PBS and fresh serum-free DMEM was added. Cells were then incubated at 37 °C in air with 5% CO2 for 0, 1, 4 and 18 h, after which bacterial viability was assessed as follows. At each harvest time, cells were washed three times with PBS, and incubated for 5 min in 1 ml of 0.01% BSA in water to lyse macrophages. Wells were scraped with a sterile rubber syringe stopper and pipetted into tubes. Lysates were heated at 65 °C for 1 h to kill germinated spores before 10-fold serial dilutions were prepared for colony counts on TSA plates. Data are presented as the number of spores per macrophage (cfu/macrophage) at the initial timepoint (t0) and the percent survival of spores within macrophages at a given time point (tx) compared to a sample taken at t0. Within each experiment, three samples of each strain were heat-treated and counted, and the geometric mean cfu for like samples was determined.
2.12. Statistical evaluations
Differences in percent survival and mean-time-to-death (MTD) for mice challenged subcutaneously with Sterne spores following different immunization schemes were determined by Fisher’s Exact Test and Kaplan–Meier Analysis, respectively. Differences in antibody titers generated by immunization were determined by one-way analysis of variance (ANOVA) followed by Tukey’s pairwise post hoc comparisons. Fisher’s Exact Test was used to analyze differences in outcomes for mice immunized with PA alone versus PA plus additional antigen(s) among low PA responders. To compare differences in germination rates among strains, changes in log10 counts of bacteria from t0 to tx were analyzed by a repeated measures ANOVA followed by one-way ANOVA and Tukey’s pairwise post hoc comparisons. Differences in spore uptake by macrophages and differences in percent survival of phagocytosed spores following incubation with different antibodies were compared by one-way ANOVA followed by Tukey’s pairwise post hoc comparisons.
3. Results
3.1. Screening spore antigen candidates for recognition by anti-spore polyclonal antibody
An examination of the existing literature revealed a list of more than 30 proteins encoded by the B. anthracis genome for which there was proteomic evidence that indicates localization of those molecules to the exosporium (Table 1; [32,33,43–48]). Therefore, we screened this group of proteins for recognition by the anti-spore polyclonal serum 311001-01. We reasoned that such recognition by antiserum raised against whole, intact spore would suggest the presence of that antigen at or near the spore surface and warrant further investigation of that protein as a possible vaccine target. For that purpose, individual B. anthracis genes were cloned into the inducible expression vector pET15b and expressed in E. coli BL21(DE3) pLysS. Following purification of each recombinant protein via the N-terminal His-tag encoded on pET15b, proper expression was confirmed by Western blot analysis using His-Tag monoclonal antibody (data not shown). Proteins were then screened by Western blot for recognition by anti-spore serum 311001-01 (Fig. 1a). Of the 31 candidates so screened, a total of 11 proteins demonstrated recognition by the anti-spore antiserum; these 11 included the immunodominant exosporium component BclA (BAS1130). The data we generated that concerned BclA as vaccine candidate were previously published [34], so BclA as an immunogen was not further examined as a part of this study.
Fig. 1.
Detection of B. anthracis spore proteins by Western blot analysis. (a) Incubation of purified His-tagged proteins (100 ng/lane) with anti-spore serum 311001-01. (b) Incubation of SSPE (5 μl/lane) prepared from Sterne or ΔbclA spores with affinity-purified anti-spore protein polyclonal IgG. Molecular weight standards (kDa) are shown on the left of each figure.
3.2. Localization of spore proteins
After identifying the spore proteins that were reactive to anti-spore polyclonal serum, polyclonal antiserum was prepared in rabbits against each of the recombinant proteins. Six of the proteins [CotZ1 (BAS1141), SOD15 (BAS1378), ExsD (BAS2439), a ThiJ/PfpI family protein (BAS4383) and here-to-for hypothetical proteins p2377 (BAS2377) and p3957 (BAS3957)] demonstrated poor solubility resulting in low levels of purified protein that initially precluded efficient immunization. In the case of the superoxide dismutase SOD15 (BAS1378), solubility was improved by cloning and expressing a truncated form of the protein, minus the first 96 amino acids at the N-terminus of the protein. This Δ1–96 form of SOD15 was detected in prior proteomic analysis of the exosporium [32] and is likely the more relevant form for further study. Along with SOD15, recognition-positive candidates BxpB (BAS1144), SODA1 (BAS4177), YwdL (BAS5242), and the hypothetical protein p5303 (BAS5303) were used for rabbit immunizations. The resulting antisera, which were purified against a Protein G column to isolate the IgG fractions, were further purified by affinity to the immunization target. Affinity-purified anti-spore protein polyclonal IgGs were then used to demonstrate the presence of the target proteins in SSPE prepared from wild-type Sterne and isogenic ΔbclA strain spores (Fig. 1b). These Western blots demonstrated that not only were the five proteins of interest part of the whole-spore immunome, but detectable levels of each protein could be found associated with the outer structures of the spore. Furthermore, while there appeared to be a shift in the form(s) of BxpB and p5303 present on the spore in the absence of BclA, none of the proteins appeared dependent on BclA for localization to the spore exterior.
3.3. Antigens near the spore surface are partially obscured by the presence of BclA
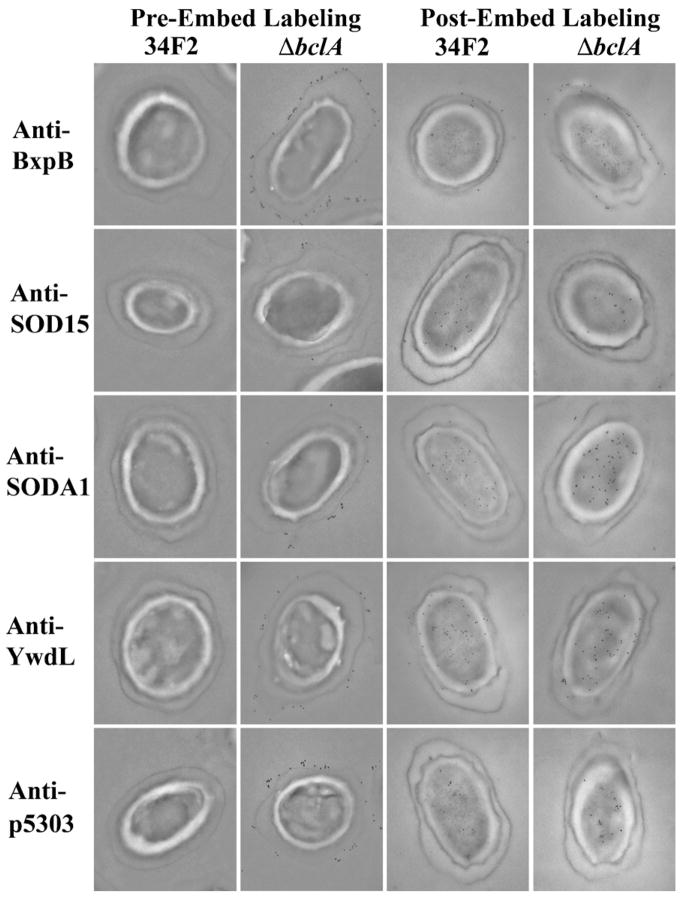
To further examine the localization of the five spore antigens identified during the screening process, spores were incubated with antigen-specific polyclonal IgG both by ELISA (Fig. 2) and by immunoelectron microscopy (Fig. 3). Recognition of the antibody targets in the context of intact Sterne spores was low when the antibodies were incubated with spore-coated ELISA plates, an observation likely attributable to the presence of BclA. The densely packed BclA proteins that are responsible for the “hair-like nap” found on the surface of the spore [33] may cause a stearic hindrance that interferes with the recognition of targets located on the spore. However, the use of spores made from an isogenic ΔbclA strain that lacks the obstructive BclA demonstrated that all five proteins could in fact be recognized by the appropriate antibodies in the context of whole spores (Fig. 2). Immunoelectron microscopy samples in which spores were labeled with antibody before embedding confirmed these observations, as recognition of antiserum targets was limited to ΔbclA spores. When antiserum staining was done after spores were first embedded and sectioned, a method that should expose epitopes buried within the structure of the spore, antigens could be identified throughout the spore. Together these data obtained by different embedding methods suggest that while the five proteins are present within the basal structures of the exosporium, they are not localized specifically to that region of the spore. One notable exception to this conclusion was the protein BxpB, a structural protein known to help anchor BclA to the exosporium [49]. Whereas other proteins appeared to be present throughout the spore with no discrete localization to the exosporium, the nearly exclusive presence of BxpB at or near the surface of the spore indicated that this protein was specifically localized to the exosporium basal surface.
Fig. 2.
Accessibility of B. anthracis surface-exposed spore proteins by ELISA. Binding of affinity-purified anti-spore protein polyclonal IgG to wells of microtiter plates coated with 1 × 107 cfu/well of either intact Sterne or ΔbclA spores was assessed by ELISA.
Fig. 3.
Localization of B. anthracis spore proteins within the spore by immunoelectron microscopy. Spores were labeled with immunogold by incubation with affinity-purified anti-spore protein polyclonal IgG followed by a gold-labeled secondary antibody. Labeling occurred either before or following embedding in 4% formaldehyde in PBS (pH 7.0) for a minimum of 8 h as described in Section 2.
3.4. Select spore antigens can provide enhanced protection against spore challenge
Previous studies with BclA [34,35] demonstrated that the protective effect conferred by immunization with formaldehyde-inactivated (FIS) spores [30] can be replicated with the use of individual spore components as immunogens in the A/J mouse model. However, these studies demonstrated that immunization with such spore antigens was not sufficient to provide protection but rather had to be used in combination with rPA. By immunizing with a single 50 ng dose of rPA, which we previously showed allows approximately 50% survival of mice after an otherwise uniformly lethal spore challenge [34], the protective effect of the spore antigen additive could be assessed by the extent of enhancement of protection beyond that afforded by rPA alone. Our first immunization scheme, which involved simultaneous immunization with 50 ng of rPA and 10 μg of recombinant spore antigen following an initial immunization with 10 μg of recombinant spore antigen alone, yielded no benefit compared to immunization with rPA alone (Table 2). This failure to protect despite the generation of a response to the spore antigens was likely due to the reduction in the anti-PA antibody response that was seen upon simultaneous immunization with a second antigen. Next, we immunized mice with a single 50 ng dose of rPA, followed 2 weeks later by a second immunization with 10 μg of recombinant spore protein. A preliminary experiment indicated that SOD15, SODA1 and YwdL failed to provide additional protection beyond that afforded by rPA alone (data not shown). BxpB and p5303 demonstrated positive preliminary results and were thus examined further (Table 3; Fig. 4a). The addition of single spore antigens as a second immunogen appeared to increase survival, though the improved outcomes were only statistically significant in the case of BxpB (P = 0.022). The improved survival corresponded to a robust humoral response generated against each spore antigen (Table 3; Fig. 4b and c) that was achieved without a significant diminution in the overall response of the immunization group(s) to rPA. These anti-spore protein antibody responses actually appeared to have altered the outcomes of mice that generated poor responses to rPA (Fig. 4d). Low PA responders (OD450 ≤1.5) that received BxpB or p5303 showed a better survival-to-death ratio (5:1, 10:3, respectively) compared to low PA responders that received no second antigen (1:9). Of note, when mice received BxpB and p5303 simultaneously as part of a second immunization the antibody response to BxpB (Fig. 4b) was diminished and added protection among low PA responders was not seen (Fig. 4a and d).
Table 3.
Serological responses for mice immunized sequentially with PA then BxpB and/or p5303 prior to s.c. spore challenge
| Group | Immunization
|
Mean anti-PA response (OD450)a | Mean anti-BxpB response (OD450)b | Mean anti-p5303 response (OD450)c | |
|---|---|---|---|---|---|
| Day 1 | Day 15 | ||||
| 1 | PBS | PBS | NR | NR | NR |
| 2 | PA (50 ng) | PBS | 1.64 | NR | NR |
| 3 | PA (50 ng) | BxpB (10 μg) | 1.79 | 2.23 | NR |
| 4 | PA (50 ng) | p5303 (10 μg) | 1.41 | NR | 3.16 |
| 5 | PA (50 ng) | BxpB (10 μg) + p5303 (10 μg) | 1.54 | 0.99* | 3.07 |
NR, No response (OD450 ≤0.15).
Optical density measurement at 450 nm for a 1:400 antiserum dilution. OD450 for positive control (monoclonal anti-PA antibody) was 1.65. OD450 for negative control (NRS) was 0.03.
Optical density measurement at 450 nm for a 1:800 antiserum dilution. OD450 for positive control (polyclonal anti-BxpB) was 3.45. OD450 for negative control (NRS) was 0.03.
Optical density measurement at 450 nm for a 1:3200 antiserum dilution. OD450 for positive control (polyclonal anti-p5303) was 3.24. OD450 for negative control (NRS) was 0.02.
P < 0.001 for the response of Group 5 compared to the response of Group 3.
Fig. 4.
BxpB provides enhanced protection against subcutaneous spore challenge. (a) A/J mice were sequentially injected i.p. on Day 1 with 50 ng of rPA and on Day 15 with either PBS (□) or 10 μg of recombinant spore proteins BxpB (○), p5303 (▲), or BxpB + p5303 (×). Control mice (◆) received PBS on both Days 1 and 15. The mice were challenged subcutaneously on Day 29 with 10 LD50’s of Sterne spores. The animals were then monitored for survival for 14 days after challenge. *Mice that received 10 μg of BxpB demonstrated enhanced survival (14 of 15 mice) compared to mice receiving 50 ng of rPA only (11 of 20 mice)(P = 0.022). #Mice that received 10 μg of p5303 alone (17 of 20 mice) or 10 μg of both BxpB and p5303 (14 of 20) did not demonstrate statistically significant enhanced survival (P ≥0.082). (b–d) Serological responses were tested by incubating Day 28 serum samples (b) at a 1:800 dilution on microtiter plates coated with BxpB, (c) at a 1:3200 dilution on microtiter plates coated with p5303, or (d) at a 1:400 dilution on microtiter plates coated with rPA. ELISA titers are from mice that survived (●) or died (□) following s.c. challenge. Lines indicate one standard deviation from the mean serological response for the group. *Survival among mice with a low (OD450 ≤1.5) anti-PA response was significantly greater when mice were boosted with BxpB or p5303 as compared to mice receiving only rPA (P ≤0.0022).
3.5. Anti-spore protein polyclonal IgGs do not inhibit germination
A previous report [50] indicates that polyclonal antiserum generated against whole spores is capable of reducing spore germination in vitro. These observations suggested that the protective benefit of FIS spores [30] may be due in part to a reduction of germination within the mouse mediated by antibodies against the spore. Given that the enhanced protection afforded by immunization with BxpB or p5303 was likely attributable to the generation of anti-BxpB or anti-p5303 antibodies, such antibody-mediated enhanced protection might be the result of an anti-germination effect. Consequently, we sought to determine whether these antibodies were capable of reducing germination in vitro. Spores were pre-incubated with NRS, anti-spore serum 311001-01 or antigen-specific IgG directed against individual spore-bound targets (BxpB, p5303, BclA) before incubation in 5% BHI. Only in the case of preincubation with 311001-01 was germination seen to be reduced (Fig. 5). These results indicate that any protective effect conferred by immunization with BxpB or p5303 (or BclA, as has previously been shown [34]) was not the result of a reduction in spore germination mediated by a humoral response to the respective immunogens.
Fig. 5.
Antibodies against individual spore proteins do not block germination. Heat-inactivated Sterne spores (1 × 108) were pre-incubated with anti-spore serum 311001-01 (□), anti-BclA (○), anti-BxpB (▲), anti-p5303 (×), or NRS (◆) before inoculation of 5% BHI broth. Colony counts of heat-treated samples that were taken from the cultures at various time points (tx) were plotted as log10 cfu against each time point. Error bars represent ±1 standard deviation from three separate experiments. Colony counts following heat-treatment represent ungerminated spores. *The level of germination of spores pre-incubated with 311001-01 was statistically lower than wild type at 15 min (P = 0.048) and 30 min (P = 0.019).
3.6. Anti-spore protein polyclonal IgG increases spore uptake and killing by macrophages
In the course of anthrax infection, macrophages are believed to serve as a site for both spore germination [51,52] and spore destruction [53,54]. Therefore, aside from reducing germination, an alternative mechanism by which antibodies directed at spores might assist in the immune response to infection is by acting as opsonins that increase the uptake and perhaps killing of spores in macrophages. To test this theory, we studied the effects of anti-BxpB and anti-p5303 antibodies on the opsonization and survival of spores in RAW264.7 macrophages (Fig. 6). Spores were preincubated with NRS, anti-spore serum 311001-01, anti-BxpB, or anti-p5303 prior to incubation with macrophages. Anti-BclA was included as a control, given its previously published [34] capacity to increase phagocytic uptake and facilitate enhanced protection in the mouse model. Not surprisingly, anti-spore serum 311001-01 showed the greatest increase in uptake compared to NRS, a rise of nearly five-fold, followed by anti-BclA and anti-BxpB (Fig. 6a). The opsonization effect of anti-p5303 was not significant (P = 0.272). When killing of phagocytosed spore was examined, spores opsonized with anti-spore serum 311001-01 or anti-BxpB were consistently killed more efficiently than spores incubated with NRS alone, whereas spores incubated with anti-BclA or anti-p5303 were more readily killed only at 4 h post-infection (Fig. 6b).
Fig. 6.
Antisera against individual spore proteins enhance phagocytosis and killing of spores. Heat-inactivated Sterne spores (1 × 108) were pre-incubated with anti-spore serum 311001-01, anti-BclA, anti-BxpB, anti-p5303, or NRS then diluted to 5 × 106 cfu/ml and added to (0.8–1.2) × 106 RAW264.7 cells at ~5 cfu/cell. Colony counts following heat-treatment represent ungerminated spores. (a) Opsonization of Sterne spores at time 0. *Phagocytosis of spores was significantly greater after treatment with anti-spore serum 311001-01, anti-BclA, or anti-BxpB than with NRS (P < 0.001). #Phagocytosis of spores was not significantly greater after treatment with anti-p5303 (P = 0.272). (b) Intracellular survival of spores after treatment with anti-spore serum 311001-01 (□), anti-BclA (○), anti-BxpB (▲), anti-p5303 (×), or NRS (◆).*Pre-incubation of spores with 311001-01, anti-BclA, anti-BxpB or anti-p5303 lead to increased killing inside macrophages after 4 h compared to pre-incubation with NRS (P ≤0.004). **Pre-incubation of spores with anti-spore pAb and anti-BxpB led to increased macrophage killing (P ≤0.002) at 1 and 18 h. Pre-incubation with anti-BclA or anti-p5303 does not lead to a significant increase in killing (P ≥ 0.058) at 1 and 18 h.
4. Discussion
In this study, we sought to identify spore antigens capable of enhancing the protective immune response conferred by recombinant protective antigen (rPA) against anthrax in an A/J mouse model. B. anthracis proteins for which there was evidence of exosporium localization were individually cloned, expressed in recombinant form, and screened for recognition by rabbit polyclonal antiserum generated against inactivated whole spores (311001-01). Recognition-positive candidates were shown to be present on the basal surface of the exosporium, where they were partially obscured by the presence of the immunodominant antigen BclA. Two of these antigens, the exosporium structural component BxpB and a previously hypothetical protein p5303 for which there is no assigned putative function, enhanced the survival of mice previously immunized with suboptimal amounts of rPA. The mechanism of enhanced protection afforded by anti-BxpB or anti-p5303 IgG was likely revealed by the observations that while these antibodies failed to reduce spore germination they did enhance the uptake of spores by RAW264.6 macrophages as well as the subsequent killing of phagocytosed spores. In the case of both enhanced protection and enhanced phagocytic uptake, the effects seen in targeting p5303 were intriguing but not statistically significant.
A growing body of evidence suggests that spores and spore antigens are potential contributors to a protective immune response against B. anthracis challenge. Attenuated live spore vaccines are capable of generating antibodies against the spore body as well as the toxins they elaborate upon germination [24,25,27,28], thereby eliciting a multi-dimensional immune response against different phases of anthrax infection. The existence of a live spore anthrax vaccine for humans, developed by scientists in the former Soviet Union, provides an ultimate confirmation of the utility of this concept [28]. Vaccines based upon inactivated spores, which are incapable of germinating and expressing toxin, can achieve similar responses when combined with the administration of exogenous PA [30,42,55]. However, concerns about safety preclude the use of viable spores in future vaccines. Therefore, any spore-based vaccine strategies that seek to recapitulate the protective benefit of whole spore immunization will require the use of acellular formulations comprised of spore subcomponents.
While the anti-PA antibodies generated by the current AVA vaccine are thought to confer protection primarily via prevention of anthrax intoxication, conflicting reports exist regarding the anti-spore capacity of antibodies directed against PA. Certain studies indicate a role for anti-PA antibodies in facilitating phagocytosis and inhibiting germination [41,56], while other studies fail to see such effects [34]. On the other hand, the protective benefit of an anti-spore immune response is presumably dependent on an impairment of spore-centric steps essential to the onset of infection. Recent studies employing a variety of approaches suggest that by targeting individual proteins localized to the spore it is possible to achieve a delay in the onset of disease and/or protection against infection [34,35,41,50,56,57]. Antibodies directed against spore components hamper germination [34,42,50,56,57], increase phagocytic uptake [56–58] and/or accelerate phagocyte-mediated spore destruction [41,56,57]. Each of these actions would result in the effective reduction of the spore inoculum and could delay the establishment of infection. Combined with a spore-induced activation of the cell-mediated immune response that leads to heightened host immune defenses [55], a delay in vegetative outgrowth would allow the host to limit or eliminate infection prior to the successful elaboration of essential anthrax virulence factors. The use of BclA as a partially protective immunogen in prior studies [34,35] served as a proof of concept for this strategy. The capacity of BxpB, and to a lesser extent p5303, to provide partial protection against anthrax spore challenge provides a further demonstration of the principle.
Antibodies to BxpB and p5303 enhanced phagocytic uptake of spores despite the fact that we concluded that each antigen was localized beneath the “hair-like” structures of BclA. Indeed, immunoelectron microscopy with anti-BxpB and anti-p5303 antibodies (Fig. 2b) would argue for the complete obscuration of these antigens by the projection of BclA from the surface of the exosporium, a presence that might sterically hinder access of antibodies to the structures below. However, recognition of BxpB and p5303 by antigen-specific antibodies on intact wild type spores in an ELISA format (Fig. 2a), as well as by antiserum generated against whole spores (Fig. 1), support the notion that antibodies might successfully bind to spore targets despite the presence of BclA. The most likely explanation for the contradiction in these data involves the techniques involved. Specifically, while the labeling with anti-BxpB and anti-p5303 antibodies for immunoelectron microscopy occurred against intact spores prior to embedding, and therefore should reflect the results seen in the ELISA protocol, the subsequent thin-sectioning of spores actually resulted in visualization of only of a single ring around the surface of the spore. Such a small “sampling” of the spore surface is more likely to miss targets present in low concentrations across the entirety of the spore than an ELISA directed against the whole spore body. Additionally, whereas the ELISA data suggest that the presence of BclA only partially obstructs antibody access, the additional size of the immunogold particle conjugated to antibodies used in the electron microscopy study could have reduced the likelihood of recognizing targets on the basal surface of the exosporium in the presence of BclA. Furthermore, recent evidence that a spore antigen localized to the spore cortex plays a role in antibody-directed spore opsonization [58] supports the contention that the spore ultrastructure possesses dynamic characteristics even before the onset of germination. Taken together, these observations suggest that the question of antigen visibility in the presence of BclA is more complex that one might first intuit.
Despite the protective benefits of immunization against spore components, the potentially lethal outcome of intoxication attributable to those spores that succeed in establishing infection necessitates the inclusion of PA in any anthrax vaccine formulation. Given that a sufficiently high dose of PA alone is adequate to protect A/J mice against challenge with Sterne strain spores, we assessed the protective benefits of BxpB and p5303 by immunizing with suboptimal doses of rPA. By this strategy, we were able to discern that BxpB and p5303 enhanced protection only when administered subsequent to PA, and not when given on the same schedule as PA. In fact, simultaneous immunization of rPA and spore antigens actually reduced protection (Table 2), an outcome undoubtedly related to the diminished antibody response to PA seen in the group that received simultaneous immunization when compared to the group that received rPA alone. This dampening of the anti-PA response is likely due to the difference in the relative amounts of rPA (50 ng) and spore antigen (10 μg) administered. Such a disparate ratio of antigen may have induced “antigenic competition”, a phenomenon that has been observed previously with combinatorial vaccines and which can result in the diminution of the humoral response generated against one or more of the immunogens administered [59–61]. Given the essentiality of an anti-PA antibody response in effective protection, interference with this response caused by the disproportionate presence of a second immunogen abrogated any potential added benefit of the spore antigens. When spore antigens were instead used as a second immunogen and enhancement was seen, the deleterious effect on the anti-PA response was not observed (Table 3). In fact, an examination of the anti-PA responses among mice in the different immunization groups (Fig. 4d) suggests that the presence of antibodies against spore antigens helped to protect individual mice that developed comparatively low anti-PA responses. That is, an immune response to spore components that confers the benefits discussed above appears to lower the threshold for the response to PA that is necessary to provide protection from intoxication. Given mouse-to-mouse variations in the antibody response generated upon immunization with rPA, the inclusion of spore components as vaccine additives protected mice that would otherwise have been susceptible upon infection. Alternative immunization regimens, to include varying the relative amounts of rPA and spore antigen in a simultaneous administration or administering simultaneously at different sites, might overcome the interference seen in our first attempt, thereby alleviating the need for sequential immunization.
The primary limitation of this study involves the use of the attenuated, non-encapsulated B. anthracis Sterne strain, and the C5 deficient A/J mouse strain [62]. The absence of the pXO2-encoded antiphagocytic capsule, a virulence factor that is expressed only in the vegetative stage and likely has no bearing on the mechanism of an immune response generated against the spore form of the bacteria, necessitates the use of an inbred mouse strain with an increases susceptibility to infection [63]. The compromised immune response of A/J mice makes the strain an imperfect model system, and perhaps partially explains the interference in the humoral response generated to antigens administered simultaneously. Indeed, subsequent vaccine studies with PA and spore antigens might be best undertaken with the fully virulent Ames strain and applicable mouse models. Nevertheless, we contend that the previously described strategy of reducing the effective infectious dose and providing partial protection through the targeting of spore antigens [34,35,41,50,56,57] was strengthened by the work reported here with the A/J mouse model system.
While prospective anthrax vaccines must always address the need to protect against intoxication, a multi-valent vaccine with antigens expressed in various stages of anthrax pathogenesis offers the possibility of improvement over the current PA-based formulation. A vaccine targeting multiple surface-expressed antigens in conjunction with PA provides a strategy analogous to the acellular pertussis vaccine formulation, which elicits an immune response both to the pertussis toxin and to an array of additional bacterial proteins. Given the dual life stages of B. anthracis seen by the host during infection, this strategy might incorporate immunogenic antigens from both the infectious spore and the vegetative bacillus. In this investigation, we identified a pair of spore antigens capable of inducing a partially protective humoral immune response. A combination of these and other recently identified spore-bound immunogens may provide viable candidates for a future multistage anthrax vaccine.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.vaccine.2008.07.015.
Acknowledgments
We thank Dr. Cara Olson for expert statistical analysis, Mr. Michael Flora for primer synthesis and DNA sequencing, Dr. Jill Czarnecki for polyclonal antibody generation, and Dr. Trupti Brahmbhatt for helpful discussions. This work was supported by NIH/NIAID Middle Atlantic Regional Center of Excellence grant U54 AI057168 and research funds from the United States Navy through Uniformed Services University grant # G173HS. Special thanks also to the United States Army Long-Term Health Education and Training Program for support of one of us (R. Cybulski). The opinions and assertions in this paper are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy or the Department of Defense. This research was conducted in compliance with the Animal Welfare Act. All animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Uniformed Services University.
References
- 1.Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–71. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 2.Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med. 1999;341(September 11):815–26. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 3.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, et al. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7(November 6):933–44. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedlander AM, Welkos SL, Ivins BE. Anthrax vaccines. Curr Top Microbiol Immunol. 2002;271:33–60. doi: 10.1007/978-3-662-05767-4_3. [DOI] [PubMed] [Google Scholar]
- 5.Brachman PS, Gold H, Plotkin SA, Fekety FR, Werrin M, Ingraham NR. Field evaluation of a human anthrax vaccine. Am J Public Health Nations Health. 1962;52(April 4):632–45. doi: 10.2105/ajph.52.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little SF, Ivins BE, Fellows PF, Friedlander AM. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect Immun. 1997;65(December 12):5171–5. doi: 10.1128/iai.65.12.5171-5175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molnar DM, Altenburn RA. Alterations in the biologocal activity of protective antigen of Bacillus anthracis toxin. Proc Soc Exp Biol Med. 1963 November;114:294–7. [PubMed] [Google Scholar]
- 8.Pitt ML, Little S, Ivins BE, Fellows P, Boles J, Barth J, et al. In vitro correlate of immunity in an animal model of inhalational anthrax. J Appl Microbiol. 1999;87(August 2):304. doi: 10.1046/j.1365-2672.1999.00897.x. [DOI] [PubMed] [Google Scholar]
- 9.Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, et al. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine. 2001;19(September 32):4768–73. doi: 10.1016/s0264-410x(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull PC, Leppla SH, Broster MG, Quinn CP, Melling J. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med Microbiol Immunol. 1988;177(5):293–303. doi: 10.1007/BF00189414. [DOI] [PubMed] [Google Scholar]
- 11.Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004;22(January 3–4):422–30. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Pittman PR, Leitman SF, Oro JG, Norris SL, Marano NM, Ranadive MV, et al. Protective antigen and toxin neutralization antibody patterns in anthrax vaccinees undergoing serial plasmapheresis. Clin Diagn Lab Immunol. 2005;12(June 6):713–21. doi: 10.1128/CDLI.12.6.713-721.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuveny S, White MD, Adar YY, Kafri Y, Altboum Z, Gozes Y, et al. Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun. 2001;69(May 5):2888–93. doi: 10.1128/IAI.69.5.2888-2893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedlander AM, Pittman PR, Parker GW. Anthrax vaccine: evidence for safety and efficacy against inhalational anthrax. JAMA. 1999;282(December 22):2104–6. doi: 10.1001/jama.282.22.2104. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull PC. Current status of immunization against anthrax: old vaccines may be here to stay for a while. Curr Opin Infect Dis. 2000;13(April 2):113–20. doi: 10.1097/00001432-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Farchaus JW, Ribot WJ, Jendrek S, Little SF. Fermentation, purification, and characterization of protective antigen from a recombinant, avirulent strain of Bacillus anthracis. Appl Environ Microbiol. 1998;64(March 3):982–91. doi: 10.1128/aem.64.3.982-991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikszta JA, Sullivan VJ, Dean C, Waterston AM, Alarcon JB, Dekker JP, et al. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005;191(January 2):278–88. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- 18.Peachman KK, Rao M, Alving CR, Burge R, Leppla SH, Rao VB, et al. Correlation between lethal toxin-neutralizing antibody titers and protection from intranasal challenge with Bacillus anthracis Ames strain spores in mice after transcutaneous immunization with recombinant anthrax protective antigen. Infect Immun. 2006;74(January 1):794–7. doi: 10.1128/IAI.74.1.794-797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloat BR, Cui Z. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharm Res. 2006;23(June 6):1217–26. doi: 10.1007/s11095-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 20.Ivins B, Fellows P, Pitt L, Estep J, Farchaus J, Friedlander A, et al. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine. 1995;13(December 18):1779–84. doi: 10.1016/0264-410x(95)00139-r. [DOI] [PubMed] [Google Scholar]
- 21.Fellows PF, Linscott MK, Ivins BE, Pitt ML, Rossi CA, Gibbs PH, et al. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine. 2001;19(April 23–24):3241–7. doi: 10.1016/s0264-410x(01)00021-4. [DOI] [PubMed] [Google Scholar]
- 22.Ivins BE, Pitt ML, Fellows PF, Farchaus JW, Benner GE, Waag DM, et al. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine. 1998;16(July 11–12):1141–8. doi: 10.1016/s0264-410x(98)80112-6. [DOI] [PubMed] [Google Scholar]
- 23.Ivins BE, Welkos SL, Little SF, Crumrine MH, Nelson GO. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect Immun. 1992;60(February 2):662–8. doi: 10.1128/iai.60.2.662-668.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welkos SL, Friedlander AM. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb Pathog. 1988;5(August 2):127–39. doi: 10.1016/0882-4010(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Mendelson I, Altboum Z, Kobiler D, Elhanany E, Bino T, et al. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect Immun. 2000;68(August 8):4549–58. doi: 10.1128/iai.68.8.4549-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein F, Dearmon IA, Jr, Lincoln RE, Mahlandt BG, Fernelius AL. Immunological studies of anthrox. II. Levels of immunity against Bacillus anthracis obtained with protective antigen and live vaccine. J Immunol. 1962 January;88:15–9. [PubMed] [Google Scholar]
- 27.Little SF, Knudson GB. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect Immun. 1986;52(May 2):509–12. doi: 10.1128/iai.52.2.509-512.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlyakhov EN, Rubinstein E. Human live anthrax vaccine in the former USSR. Vaccine. 1994;12(June 8):727–30. doi: 10.1016/0264-410x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 29.Turnbull PC, Broster MG, Carman JA, Manchee RJ, Melling J. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect Immun. 1986;52(May 2):356–63. doi: 10.1128/iai.52.2.356-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brossier F, Levy M, Mock M. Anthrax spores make an essential contribution to vaccine efficacy. Infect Immun. 2002;70(February 2):661–4. doi: 10.1128/iai.70.2.661-664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerhardt P, Ribi E. Ultrastructure of the exosporium enveloping spores of Bacillus cereus. J Bacteriol. 1964 December;88:1774–89. doi: 10.1128/jb.88.6.1774-1789.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steichen C, Chen P, Kearney JF, Turnbough CL., Jr Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J Bacteriol. 2003;185(March 6):1903–10. doi: 10.1128/JB.185.6.1903-1910.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sylvestre P, Couture-Tosi E, Mock M. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol Microbiol. 2002;45(July 1):169–78. doi: 10.1046/j.1365-2958.2000.03000.x. [DOI] [PubMed] [Google Scholar]
- 34.Brahmbhatt TN, Darnell SC, Carvalho HM, Sanz P, Kang TJ, Bull RL, et al. Recombinant exosporium protein BclA of Bacillus anthracis is effective as a booster for mice primed with suboptimal amounts of protective antigen. Infect Immun. 2007;75(November 11):5240–7. doi: 10.1128/IAI.00884-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn UK, Boehm R, Beyer W. DNA vaccination against anthrax in mice-combination of anti-spore and anti-toxin components. Vaccine. 2006;24:4569–71. doi: 10.1016/j.vaccine.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Brahmbhatt TN, Janes BK, Stibitz ES, Darnell SC, Sanz P, Rasmussen SB, et al. Bacillus anthracis exosporium protein BclA affects spore germination, interaction with extracellular matrix proteins, and hydrophobicity. Infect Immun. 2007;75(November 11):5233–9. doi: 10.1128/IAI.00660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanz P, Teel LD, Alem F, Carvalho HM, Darnell SC, O’Brien AD. Detection of Bacillus anthracis spore germination in vivo by bioluminescence imaging. Infect Immun. 2008;76(March 3):1036–47. doi: 10.1128/IAI.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HU, Goepfert JM. A sporulation medium for Bacillus anthracis. J Appl Bacteriol. 1974;37(June 2):265–7. doi: 10.1111/j.1365-2672.1974.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 39.Aronson AI, Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976;40(June 2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little SF, Leppla SH, Cora E. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect Immun. 1988;56(July 7):1807–13. doi: 10.1128/iai.56.7.1807-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welkos S, Friedlander A, Weeks S, Little S, Mendelson I. In-vitro characterisation of the phagocytosis and fate of anthrax spores in macrophages and the effects of anti-PA antibody. J Med Microbiol. 2002;51(October 10):821–31. doi: 10.1099/0022-1317-51-10-821. [DOI] [PubMed] [Google Scholar]
- 42.Enkhtuya J, Kawamoto K, Kobayashi Y, Uchida I, Rana N, Makino S. Significant passive protective effect against anthrax by antibody to Bacillus anthracis inactivated spores that lack two virulence plasmids. Microbiology. 2006;152(October Pt 10):3103–10. doi: 10.1099/mic.0.28788-0. [DOI] [PubMed] [Google Scholar]
- 43.Redmond C, Baillie LW, Hibbs S, Moir AJ, Moir A. Identification of proteins in the exosporium of Bacillus anthracis. Microbiology. 2004;150(February Pt 2):355–63. doi: 10.1099/mic.0.26681-0. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Bergman NH, Thomason B, Shallom S, Hazen A, Crossno J, et al. Formation and composition of the Bacillus anthracis endospore. J Bacteriol. 2004;186(January 1):164–78. doi: 10.1128/JB.186.1.164-178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Read TD, Salzberg SL, Pop M, Shumway M, Umayam L, Jiang L, et al. Comparative genome sequencing for discovery of novel polymorphisms in Bacillus anthracis. Science. 2002;296(June 5575):2028–33. doi: 10.1126/science.1071837. [DOI] [PubMed] [Google Scholar]
- 46.Charlton S, Moir AJ, Baillie L, Moir A. Characterization of the exosporium of Bacillus cereus. J Appl Microbiol. 1999;87(August 2):241–5. doi: 10.1046/j.1365-2672.1999.00878.x. [DOI] [PubMed] [Google Scholar]
- 47.Lai EM, Phadke ND, Kachman MT, Giorno R, Vazquez S, Vazquez JA, et al. Proteomic analysis of the spore coats of Bacillus subtilis and Bacillus anthracis. J Bacteriol. 2003;185(February 4):1443–54. doi: 10.1128/JB.185.4.1443-1454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Todd SJ, Moir AJ, Johnson MJ, Moir A. Genes of Bacillus cereus and Bacillus anthracis encoding proteins of the exosporium. J Bacteriol. 2003;185(June 11):3373–8. doi: 10.1128/JB.185.11.3373-3378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steichen CT, Kearney JF, Turnbough CL., Jr Characterization of the exosporium basal layer protein BxpB of Bacillus anthracis. J Bacteriol. 2005;187(September 17):5868–76. doi: 10.1128/JB.187.17.5868-5876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welkos SL, Cote CK, Rea KM, Gibbs PH. A microtiter fluorometric assay to detect the germination of Bacillus anthracis spores and the germination inhibitory effects of antibodies. J Microbiol Methods. 2004;56(February 2):253–65. doi: 10.1016/j.mimet.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 51.Dixon TC, Fadl AA, Koehler TM, Swanson JA, Hanna PC. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell Microbiol. 2000;2(December 6):453–63. doi: 10.1046/j.1462-5822.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 52.Guidi-Rontani C, Weber-Levy M, Labruyere E, Mock M. Germination of Bacillus anthracis spores within alveolar macrophages. Mol Microbiol. 1999;31(January 1):9–17. doi: 10.1046/j.1365-2958.1999.01137.x. [DOI] [PubMed] [Google Scholar]
- 53.Cote CK, Rea KM, Norris SL, Van RN, Welkos SL. The use of a model of in vivo macrophage depletion to study the role of macrophages during infection with Bacillus anthracis spores. Microb Pathog. 2004;37(October 4):169–75. doi: 10.1016/j.micpath.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Cote CK, Van RN, Welkos SL. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect Immun. 2006;74(January 1):469–80. doi: 10.1128/IAI.74.1.469-480.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glomski IJ, Corre JP, Mock M, Goossens PL. Cutting edge: IFN-γ-producing CD4 T lymphocytes mediate spore-induced immunity to capsulated Bacillus anthracis. J Immunol. 2007;178(March 5):2646–50. doi: 10.4049/jimmunol.178.5.2646. [DOI] [PubMed] [Google Scholar]
- 56.Cote CK, Rossi CA, Kang AS, Morrow PR, Lee JS, Welkos SL. The detection of protective antigen (PA) associated with spores of Bacillus anthracis and the effects of anti-PA antibodies on spore germination and macrophage interactions. Microb Pathog. 2005;38(May 5–6):209–25. doi: 10.1016/j.micpath.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Welkos S, Little S, Friedlander A, Fritz D, Fellows P. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology. 2001;147(June Pt 6):1677–85. doi: 10.1099/00221287-147-6-1677. [DOI] [PubMed] [Google Scholar]
- 58.Cote CK, Bozue J, Moody KL, Dimezzo TL, Chapman CE, Welkos SL. Analysis of a novel spore antigen in Bacillus anthracis that contributes to spore opsonization. Microbiology. 2008;154(February Pt 2):619–32. doi: 10.1099/mic.0.2007/008292-0. [DOI] [PubMed] [Google Scholar]
- 59.Ben-Efraim S, Liacopoulos P. Inhibition, no-effect or enhancement of immune responses following injection of mixtures of immunogenic and non-immunogenic synthetic polypeptides. Immunology. 1967;12(May 5):517–24. [PMC free article] [PubMed] [Google Scholar]
- 60.Schechter I. Antigenic competition between polypeptidyl determinants in normal and tolerant rabbits. J Exp Med. 1968;127(February 2):237–50. doi: 10.1084/jem.127.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dagan R, Goldblatt D, Maleckar JR, Yaich M, Eskola J. Reduction of antibody response to an 11-valent pneumococcal vaccine coadministered with a vaccine containing acellular pertussis components. Infect Immun. 2004;72(September 9):5383–91. doi: 10.1128/IAI.72.9.5383-5391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The Jackson Laboratory. A/J Strain Information Page. 2008 http://jaxmice.jax.org/strain/000646.html.
- 63.Welkos SL, Friedlander AM. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb Pathog. 1988;4(January 1):53–69. doi: 10.1016/0882-4010(88)90048-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.vaccine.2008.07.015.