Abstract
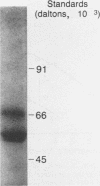
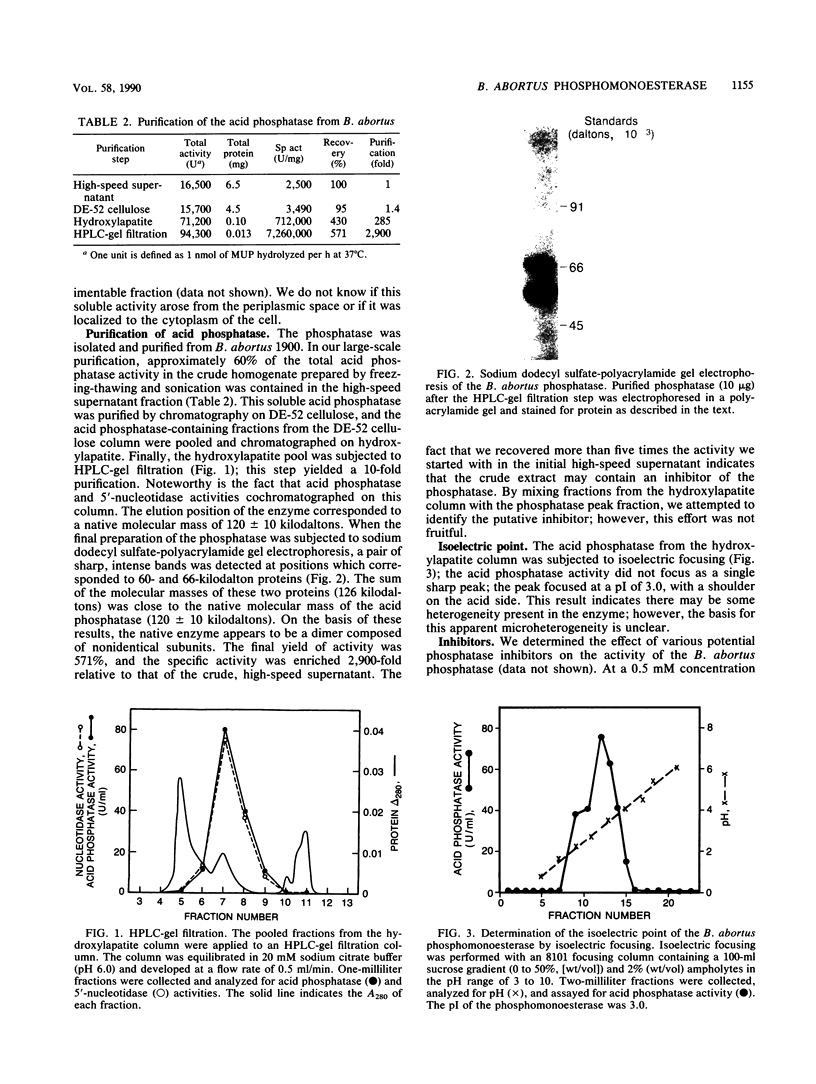
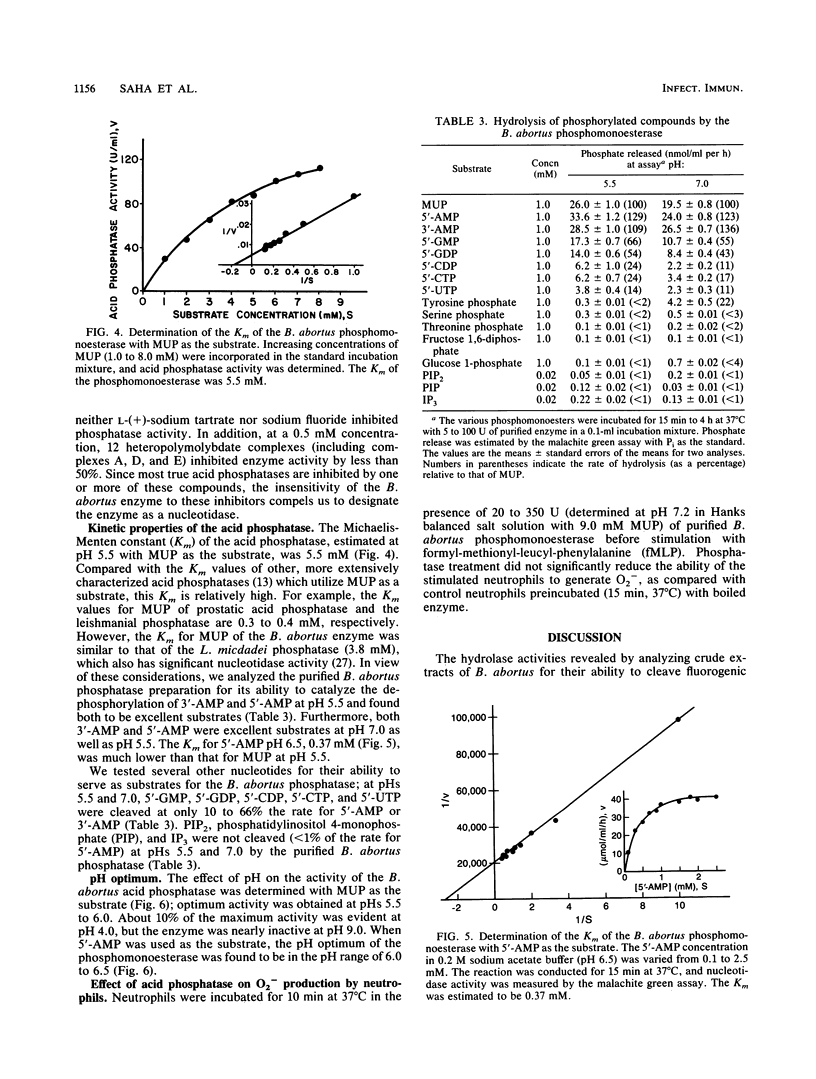
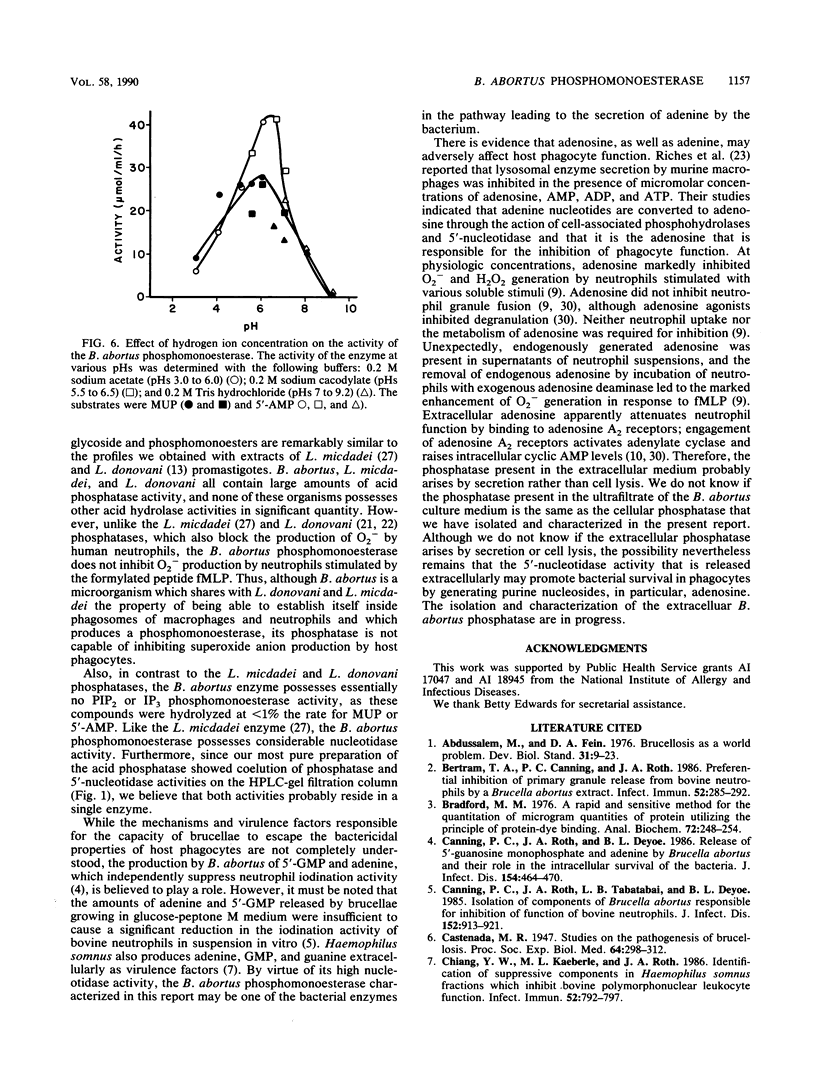
Brucellae are facultative intracellular bacterial pathogens that reside primarily in cells of the reticuloendothelial system. The high-speed supernatant obtained after centrifuging a suspension of Brucella abortus that had been frozen-thawed and sonicated contained abundant phosphomonoesterase activity, determined by using 4-methylumbelliferylphosphate as the substrate; this enzyme was purified 2,900-fold (yield, 570%) by chromatography on DE-52 cellulose and hydroxylapatite columns and high-performance liquid chromatography-gel filtration. The native enzyme had a molecular mass of 120,000 daltons (+/- 10,000 daltons), as determined by gel filtration chromatography, and resolved into two bands (60,000 and 66,000 daltons) when subjected to polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. The B. abortus phosphomonoesterase had the following properties: pH optimum, 6.0 to 6.5; isoelectric point, 3.0; substrate specificity, 5'-AMP greater than 3'-AMP greater than 3'-GMP greater than 5'-GDP greater than 5'-CDP greater than 5'-CTP greater than 5'-UPT greater than phosphotyrosine greater than phosphoserine greater than phosphothreonine. The Km for 5'-AMP was 0.37 mM. Phosphatidylinositol 4,5-bisphosphate and myo-inositol 1,3,4-trisphosphate were poor substrates for the B. abortus enzyme. The phosphomonoesterase did not inhibit superoxide anion production by human neutrophils stimulated with formyl-methionyl-leucyl-phenylalanine. The phosphomonoesterase may be one of the bacterial enzymes in the pathway leading to the production of adenine, which is secreted by B. abortus and blocks the activation of neutrophils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdussalam M., Fein D. A. Brucellosis as a world problem. Dev Biol Stand. 1976;31:9–23. [PubMed] [Google Scholar]
- Bertram T. A., Canning P. C., Roth J. A. Preferential inhibition of primary granule release from bovine neutrophils by a Brucella abortus extract. Infect Immun. 1986 Apr;52(1):285–292. doi: 10.1128/iai.52.1.285-292.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canning P. C., Roth J. A., Deyoe B. L. Release of 5'-guanosine monophosphate and adenine by Brucella abortus and their role in the intracellular survival of the bacteria. J Infect Dis. 1986 Sep;154(3):464–470. doi: 10.1093/infdis/154.3.464. [DOI] [PubMed] [Google Scholar]
- Canning P. C., Roth J. A., Tabatabai L. B., Deyoe B. L. Isolation of components of Brucella abortus responsible for inhibition of function in bovine neutrophils. J Infect Dis. 1985 Nov;152(5):913–921. doi: 10.1093/infdis/152.5.913. [DOI] [PubMed] [Google Scholar]
- Chiang Y. W., Kaeberle M. L., Roth J. A. Identification of suppressive components in "Haemophilus somnus" fractions which inhibit bovine polymorphonuclear leukocyte function. Infect Immun. 1986 Jun;52(3):792–797. doi: 10.1128/iai.52.3.792-797.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Rosenstein E. D., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985 Aug;135(2):1366–1371. [PubMed] [Google Scholar]
- Das S., Saha A. K., Remaley A. T., Glew R. H., Dowling J. N., Kajiyoshi M., Gottlieb M. Hydrolysis of phosphoproteins and inositol phosphates by cell surface phosphatase of Leishmania donovani. Mol Biochem Parasitol. 1986 Aug;20(2):143–153. doi: 10.1016/0166-6851(86)90026-5. [DOI] [PubMed] [Google Scholar]
- Ficht T. A., Bearden S. W., Sowa B. A., Adams L. G. A 36-kilodalton Brucella abortus cell envelope protein is encoded by repeated sequences closely linked in the genomic DNA. Infect Immun. 1988 Aug;56(8):2036–2046. doi: 10.1128/iai.56.8.2036-2046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Czuczman M. S., Diven W. F., Berens R. L., Pope M. T., Katsoulis D. E. Partial purification and characterization of particulate acid phosphatase of Leishmania donovani promastigotes. Comp Biochem Physiol B. 1982;72(4):581–590. doi: 10.1016/0305-0491(82)90510-7. [DOI] [PubMed] [Google Scholar]
- Kreutzer D. L., Dreyfus L. A., Robertson D. C. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):737–742. doi: 10.1128/iai.23.3.737-742.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Role of a major outer membrane protein in Escherichia coli. J Bacteriol. 1977 Aug;131(2):631–637. doi: 10.1128/jb.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert M., Andrews P. C., Babior B. M. Measurement of O2- production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 1984;105:358–365. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974 Sep;130(3):300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Harcus J. L., Symes P. H., Romito R., Donowitz G. R. Failure of the phagocytic oxidative response to protect human monocyte-derived macrophages from infection by Leishmania donovani. J Immunol. 1982 Sep;129(3):1282–1286. [PubMed] [Google Scholar]
- Remaley A. T., Glew R. H., Kuhns D. B., Basford R. E., Waggoner A. S., Ernst L. A., Pope M. Leishmania donovani: surface membrane acid phosphatase blocks neutrophil oxidative metabolite production. Exp Parasitol. 1985 Dec;60(3):331–341. doi: 10.1016/0014-4894(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Remaley A. T., Kuhns D. B., Basford R. E., Glew R. H., Kaplan S. S. Leishmanial phosphatase blocks neutrophil O-2 production. J Biol Chem. 1984 Sep 25;259(18):11173–11175. [PubMed] [Google Scholar]
- Riches D. W., Watkins J. L., Henson P. M., Stanworth D. R. Regulation of macrophage lysosomal secretion by adenosine, adenosine phosphate esters, and related structural analogues of adenosine. J Leukoc Biol. 1985 May;37(5):545–557. doi: 10.1002/jlb.37.5.545. [DOI] [PubMed] [Google Scholar]
- Riley L. K., Robertson D. C. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun. 1984 Oct;46(1):224–230. doi: 10.1128/iai.46.1.224-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. B., Glew R. H. A tartrate-resistant acid phosphatase from Gaucher spleen. Purification and properties. J Biol Chem. 1980 Jun 25;255(12):5864–5870. [PubMed] [Google Scholar]
- Saha A. K., Das S., Glew R. H., Gottlieb M. Resistance of leishmanial phosphatases to inactivation by oxygen metabolites. J Clin Microbiol. 1985 Sep;22(3):329–332. doi: 10.1128/jcm.22.3.329-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A. K., Dowling J. N., LaMarco K. L., Das S., Remaley A. T., Olomu N., Pope M. T., Glew R. H. Properties of an acid phosphatase from Legionella micdadei which blocks superoxide anion production by human neutrophils. Arch Biochem Biophys. 1985 Nov 15;243(1):150–160. doi: 10.1016/0003-9861(85)90783-0. [DOI] [PubMed] [Google Scholar]
- Saha A. K., Dowling J. N., Mukhopadhyay N. K., Glew R. H. Demonstration of two protein kinases in extracts of Legionella micdadei. J Gen Microbiol. 1988 May;134(5):1275–1281. doi: 10.1099/00221287-134-5-1275. [DOI] [PubMed] [Google Scholar]
- Saha A. K., Dowling J. N., Pasculle A. W., Glew R. H. Legionella micdadei phosphatase catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate in human neutrophils. Arch Biochem Biophys. 1988 Aug 15;265(1):94–104. doi: 10.1016/0003-9861(88)90375-x. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Imre K. M. The effects of adenosine agonists on human neutrophil function. J Immunol. 1986 Nov 15;137(10):3284–3289. [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Weinbaum D. L., Bailey J., Benner R. R., Pasculle A. W., Dowling J. N. The contribution of human neutrophils and serum to host defense against Legionella micdadei. J Infect Dis. 1983 Sep;148(3):510–517. doi: 10.1093/infdis/148.3.510. [DOI] [PubMed] [Google Scholar]