Abstract
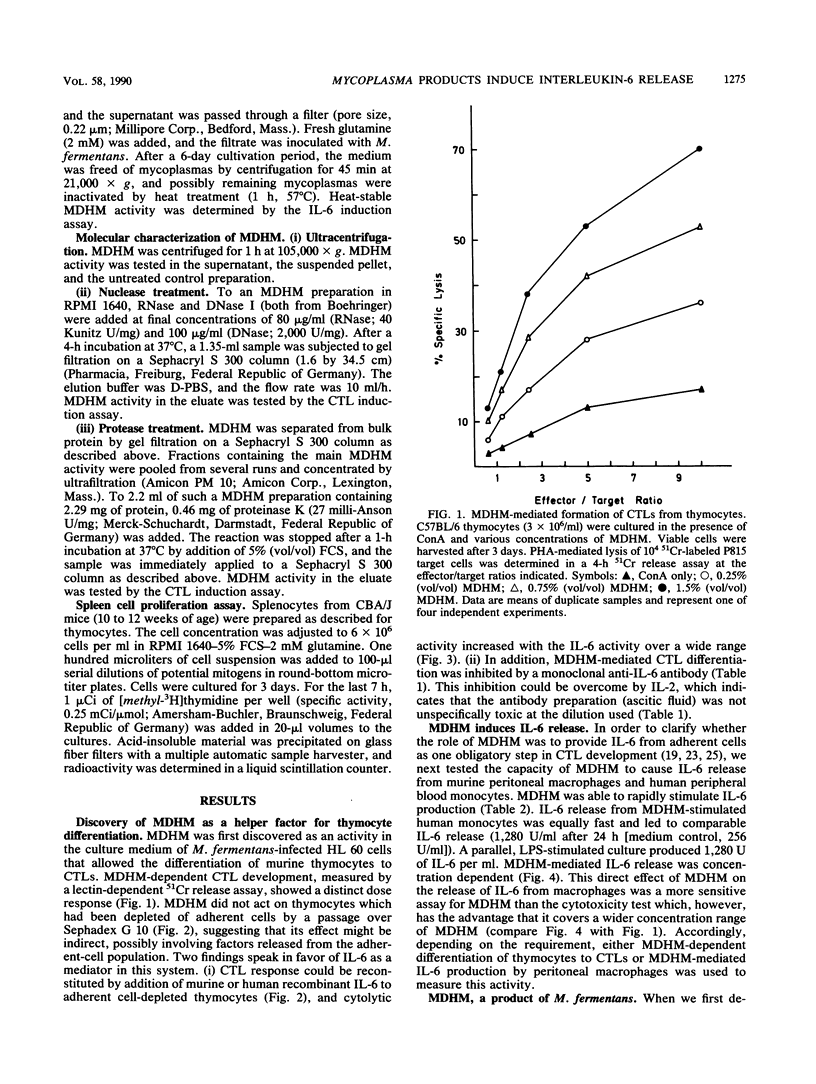
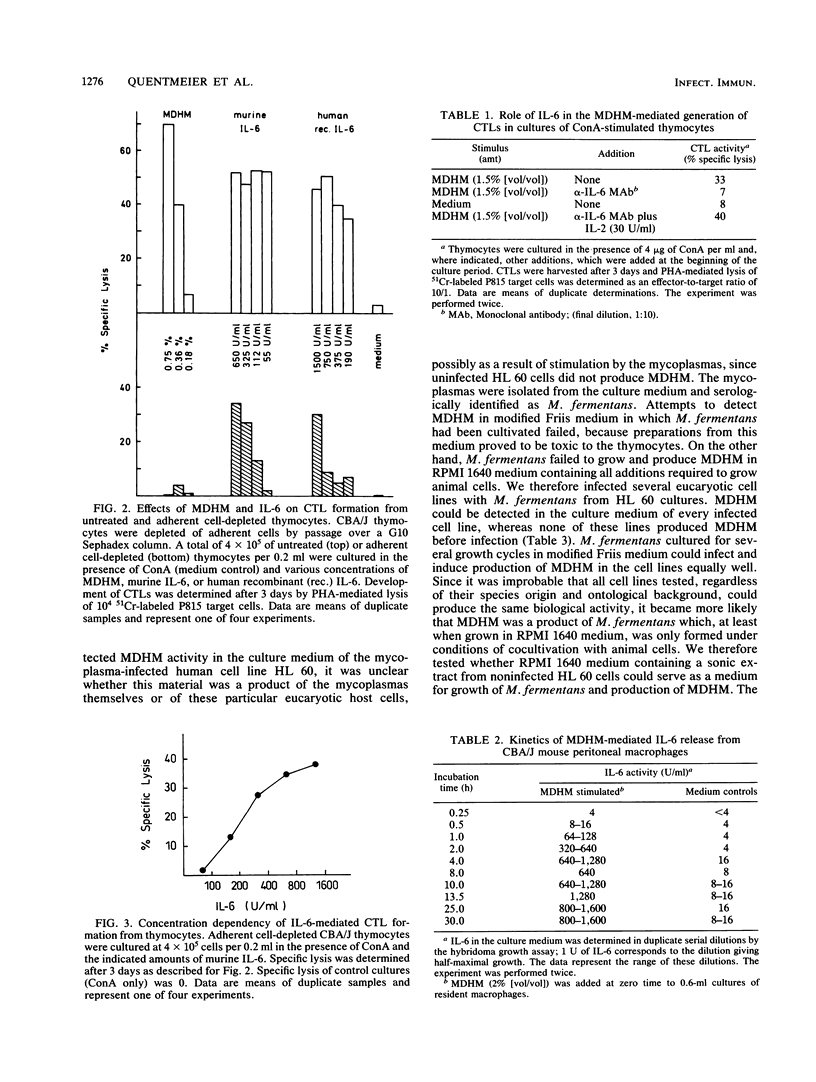
A Mycoplasma fermentans-derived high-molecular-weight material (MDHM) is described which causes differentiation of concanavalin A-stimulated CBA/J or C57BL/6 mouse thymocytes to cytolytic effector T cells (CTLs). The effect of MDHM was inhibited by addition of monoclonal anti-interleukin-6 (IL-6) antibody. It could also be abolished after removal of adherent cells. However, adherent cell-depleted thymocytes could still form CTLs after addition of IL-6. The action of MDHM could thus be explained by the capacity of MDHM to stimulate IL-6 release from adherent cells. MDHM was active on macrophages from CBA/J and C3H/HeJ endotoxin nonresponder mice and was also capable of stimulating IL-6 release from human monocytes. On gel chromatography, MDHM had an apparent molecular size of 1.5 x 10(6) daltons. Treatment with RNase and DNase had no effect on either size or biological activity. Proteinase K did not abolish activity but reduced the apparent molecular size of MDHM. MDHM production by M. fermentans required either coculture with eucaryotic cell lines in RPMI 1640 medium with fetal calf serum or addition of eucaryotic cell sonic extracts to this medium. The biological activity of MDHM is not identical to that of a mitogen for murine spleen cells derived from M. arthritidis; MDHM caused only slight proliferation in this system compared with the mitogen from M. arthritidis, and the latter did not elicit IL-6 release from macrophages. The results are discussed in relation to mycoplasmas as putative etiological agents for rheumatoid arthritis, since high IL-6 titers were reported for synovial fluid from patients with this disease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Cole B. C., Sullivan G. J., Washburn L. R., Wiley B. B. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. V. A small basic protein from culture supernatants is a potent T cell mitogen. J Immunol. 1986 Sep 1;137(5):1581–1589. [PubMed] [Google Scholar]
- Bennett J. C. The infectious etiology of rheumatoid arthritis. New considerations. Arthritis Rheum. 1978 Jun;21(5):531–538. doi: 10.1002/art.1780210507. [DOI] [PubMed] [Google Scholar]
- Borish L., Rosenbaum R., Albury L., Clark S. Activation of neutrophils by recombinant interleukin 6. Cell Immunol. 1989 Jul;121(2):280–289. doi: 10.1016/0008-8749(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Aldridge K. E., Ward J. R. Mycoplasma-dependent activation of normal lymphocytes: mitogenic potential of mycoplasmas for mouse lymphocytes. Infect Immun. 1977 Nov;18(2):393–399. doi: 10.1128/iai.18.2.393-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Cassell G. H. Mycoplasma infections as models of chronic joint inflammation. Arthritis Rheum. 1979 Dec;22(12):1375–1381. doi: 10.1002/art.1780221209. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981 Nov;127(5):1931–1936. [PubMed] [Google Scholar]
- Cole B. C., Tuller J. W., Sullivan G. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. VI. Detection of a non-MHC gene(s) in the E alpha-bearing RIIIS mouse strain that is associated with a specific lack of T cell responses to the M. arthritidis soluble mitogen. J Immunol. 1987 Aug 1;139(3):927–935. [PubMed] [Google Scholar]
- Cunha-Melo J. R., Dean N. M., Moyer J. D., Maeyama K., Beaven M. A. The kinetics of phosphoinositide hydrolysis in rat basophilic leukemia (RBL-2H3) cells varies with the type of IgE receptor cross-linking agent used. J Biol Chem. 1987 Aug 25;262(24):11455–11463. [PubMed] [Google Scholar]
- Del Giudice R. A., Robillard N. F., Carski T. R. Immunofluorescence identification of Mycoplasma on agar by use of incident illumination. J Bacteriol. 1967 Apr;93(4):1205–1209. doi: 10.1128/jb.93.4.1205-1209.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis N. F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975 Jun;27(6):337–339. [PubMed] [Google Scholar]
- Giebler D., Brehm G., Nicklas W., Kirchner H. Activation of C57BL/6 spleen cells by the mitogenic principle derived from mycoplasma arthritidis. Immunobiology. 1986 Mar;171(1-2):57–66. doi: 10.1016/S0171-2985(86)80017-1. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Houssiau F. A., Devogelaer J. P., Van Damme J., de Deuxchaisnes C. N., Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988 Jun;31(6):784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Bauer A., Moritz T., Herbst F. Lymphocyte activation and induction of interferon gamma in human leucocyte cultures by the mitogen in Mycoplasma arthritidis supernatant (MAS). Scand J Immunol. 1986 Nov;24(5):609–613. doi: 10.1111/j.1365-3083.1986.tb02177.x. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Brehm G., Nicklas W., Beck R., Herbst F. Biochemical characterization of the T-cell mitogen derived from Mycoplasma arthritidis. Scand J Immunol. 1986 Sep;24(3):245–249. doi: 10.1111/j.1365-3083.1986.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Monner D. A. An assay for growth of mouse bone marrow cells in microtiter liquid culture using the tetrazolium salt MTT, and its application to studies of myelopoiesis. Immunol Lett. 1988 Dec;19(4):261–268. doi: 10.1016/0165-2478(88)90152-6. [DOI] [PubMed] [Google Scholar]
- Mårdh P. A., Nilsson F. J., Bjelle A. Mycoplasmas and bacteria in synovial fluid from patients with arthritis. Ann Rheum Dis. 1973 Jul;32(4):319–325. doi: 10.1136/ard.32.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Tsai H., Conradt P. Effects of pyocyanine, a blue pigment from Pseudomonas aeruginosa, on separate steps of T cell activation: interleukin 2 (IL 2) production, IL 2 receptor formation, proliferation and induction of cytolytic activity. Eur J Immunol. 1986 Apr;16(4):434–440. doi: 10.1002/eji.1830160421. [DOI] [PubMed] [Google Scholar]
- Okada M., Kitahara M., Kishimoto S., Matsuda T., Hirano T., Kishimoto T. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol. 1988 Sep 1;141(5):1543–1549. [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Shinomiya H., Nakano M. Calcium ionophore A23187 does not stimulate lipopolysaccharide nonresponsive C3H/HeJ peritoneal macrophages to produce interleukin 1. J Immunol. 1987 Oct 15;139(8):2730–2736. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Wong G. G., Clark S. C., Burakoff S. J., Herrmann S. H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):508–512. [PubMed] [Google Scholar]
- Teh H. S., Ho M., Williams L. D. Suppression of cytotoxic responses by a supernatant factor derived from Mycoplasma hyorhinis-infected mammalian cell lines. Infect Immun. 1988 Jan;56(1):197–203. doi: 10.1128/iai.56.1.197-203.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Pike S. E. Interferon-beta 2/interleukin 6 is a co-stimulant for human T lymphocytes. J Immunol. 1988 Sep 1;141(5):1556–1562. [PubMed] [Google Scholar]
- Van Damme J., Van Beeumen J., Decock B., Van Snick J., De Ley M., Billiau A. Separation and comparison of two monokines with lymphocyte-activating factor activity: IL-1 beta and hybridoma growth factor (HGF). Identification of leukocyte-derived HGF as IL-6. J Immunol. 1988 Mar 1;140(5):1534–1541. [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink A., Coulie P. G., Wauters P., Nordan R. P., Van Snick J. B cell growth and differentiation activity of interleukin-HP1 and related murine plasmacytoma growth factors. Synergy with interleukin 1. Eur J Immunol. 1988 Apr;18(4):607–612. doi: 10.1002/eji.1830180418. [DOI] [PubMed] [Google Scholar]
- Waage A., Kaufmann C., Espevik T., Husby G. Interleukin-6 in synovial fluid from patients with arthritis. Clin Immunol Immunopathol. 1989 Mar;50(3):394–398. doi: 10.1016/0090-1229(89)90146-3. [DOI] [PubMed] [Google Scholar]
- Williams M. H., Brostoff J., Roitt I. M. Possible role of Mycoplasma fermentans in pathogenesis of rheumatoid arthritis. Lancet. 1970 Aug 8;2(7667):277–280. doi: 10.1016/s0140-6736(70)91328-0. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]


