Abstract
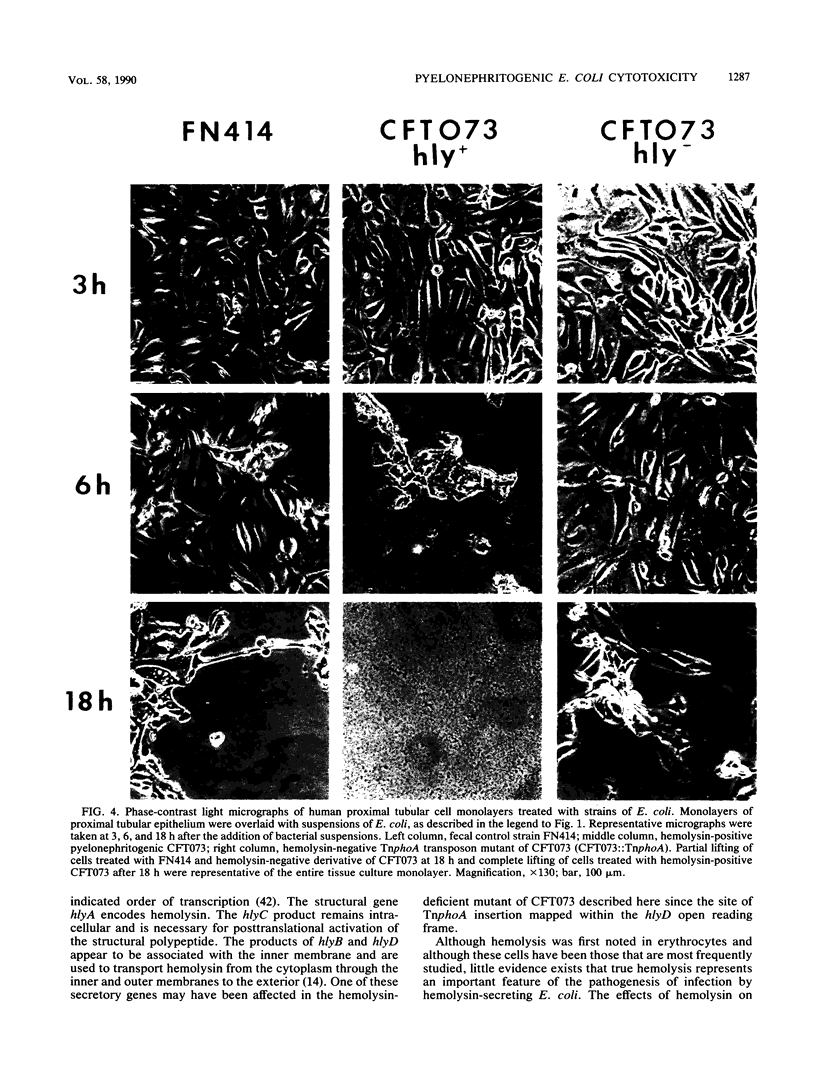
Acute pyelonephritis, a complication of Escherichia coli bacteriuria, must represent a bacterial invasion through the kidney epithelium. To study this process, we overlaid bacterial suspensions onto monolayers of cultured human kidney proximal tubular epithelial cells and measured cytotoxicity by release of lactate dehydrogenase (LDH). Thirty-four isolates cultured from patients with acute pyelonephritis were screened for the ability to cause pyelonephritis in CBA mice by transurethral challenge. The eight most virulent strains (greater than or equal to 70% of mice challenged developed greater than or equal to 10(3) CFU/g of kidney after 48 h) were selected for study. Each strain displayed mannose-resistant hemagglutination of human O erythrocytes; three strains were phenotypically and genotypically hemolytic. Pyelonephritogenic strains were significantly more cytotoxic (30.1 +/- 9.5% LDH release after 18 h) than eight fecal control strains (13.5 +/- 11.5% LDH release; P = 0.0068). We selected the most cytotoxic strain, CFT073, for further study. Sterile filtrate from this hemolytic strain was significantly more cytotoxic than was the filtrate of the fecal control strain, FN414. Transposon mutagenesis of CFT073 with TnphoA abolished hemolytic activity and cytotoxicity by both whole cells and sterile filtrate. Southern blot analysis revealed that the Tnphoa insertion mapped to the E. coli chromosomal hly determinant within a 12-kilobase SalI restriction fragment. Transformation of a nonhemolytic strain, CPZ005 with plasmid pSF4000, which carries a cloned hemolysin determinant, resulted in highly elevated cytotoxicity. Light micrographs of proximal tubular epithelial cell cultures demonstrated cell damage by pyelonephritogenic strains that was not induced by a fecal strain or the hemolysin-deficient mutant. Results indicate that pyelonephritogenic E. coli strains are more frequently cytotoxic for a putative target, that is, human renal tubular epithelium, than are fecal isolates. Hemolysin, in some strains, is apparently responsible for this cytotoxicity.
Full text
PDF


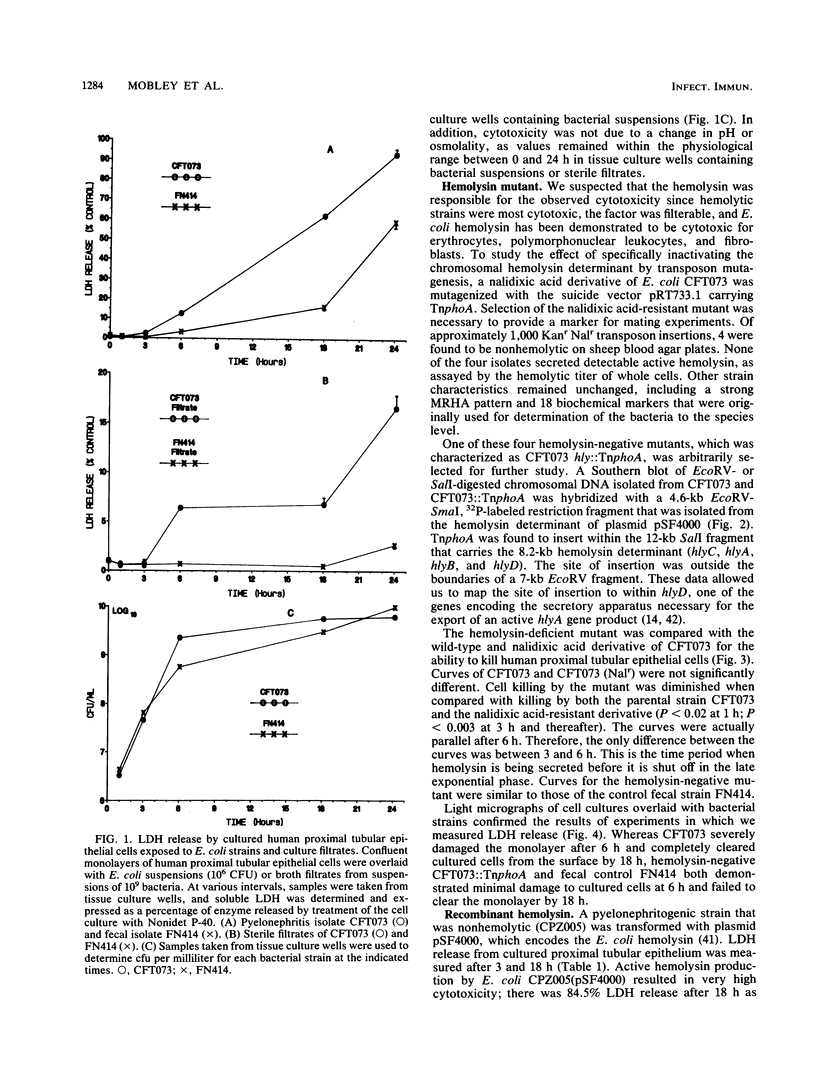
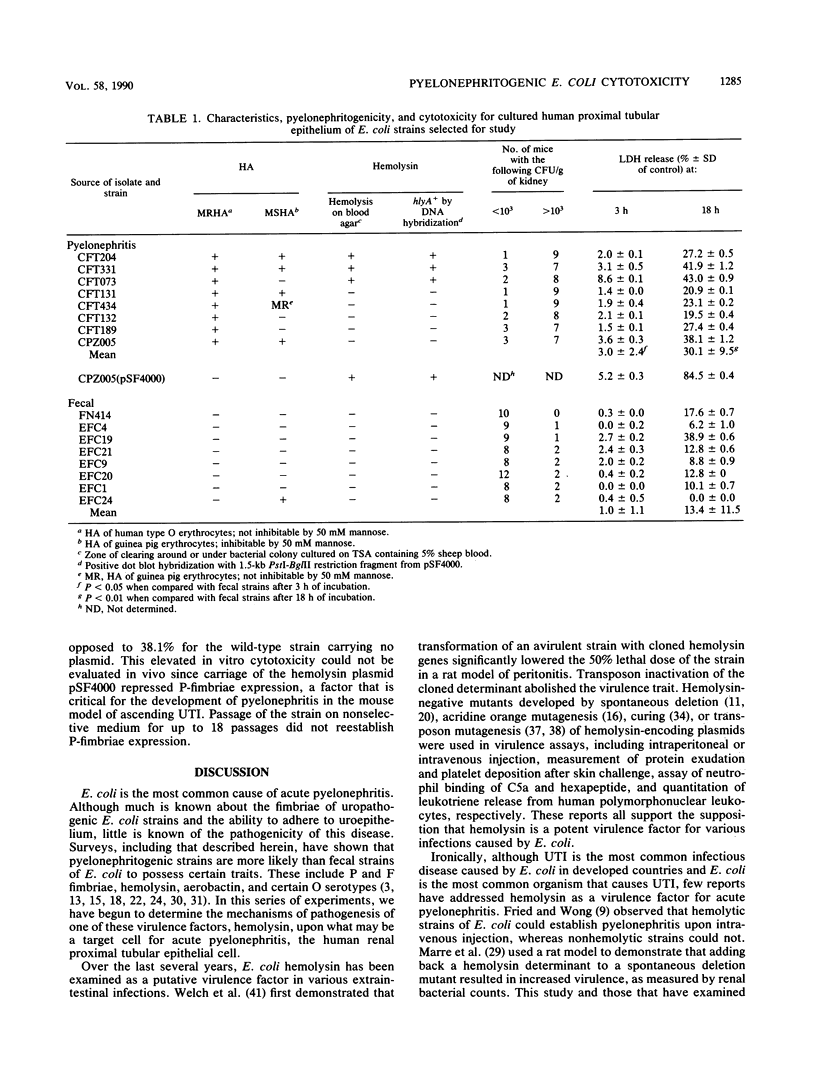
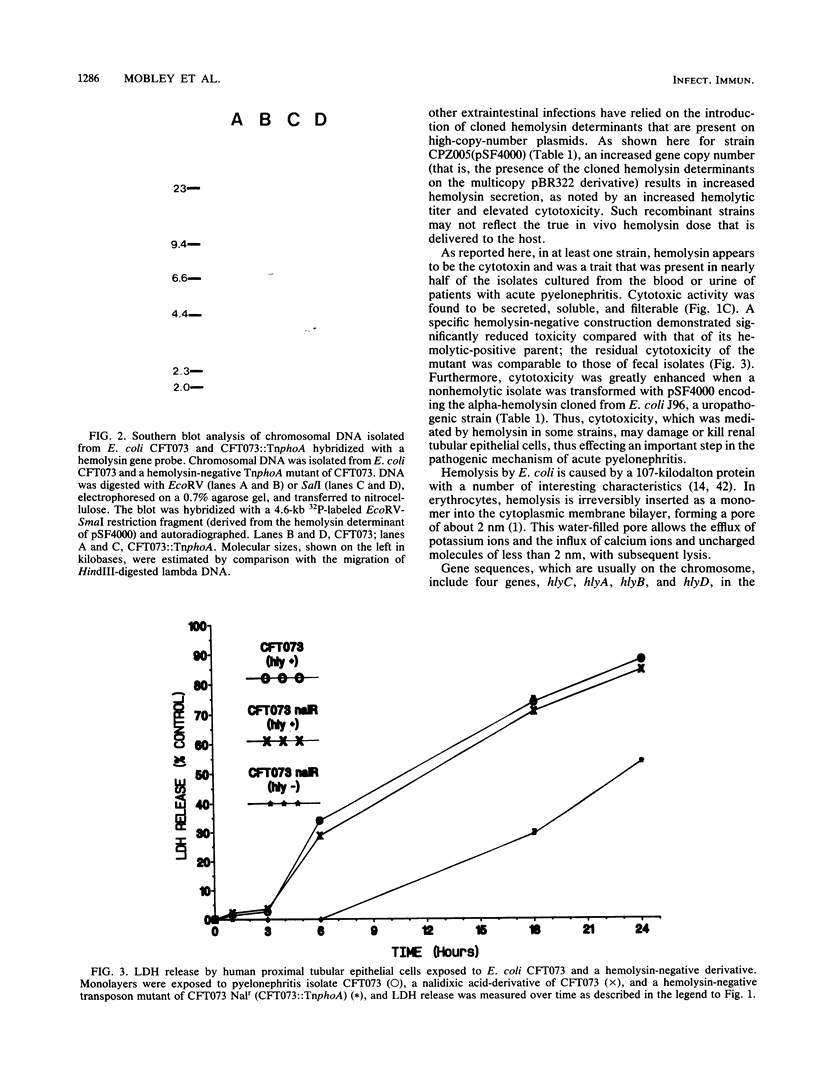


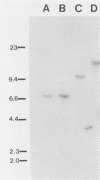
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks H. J., O'Grady F., McSherry M. A., Cattell W. R. Uropathogenic properties of Escherichia coli in recurrent urinary-tract infection. J Med Microbiol. 1980 Feb;13(1):57–68. doi: 10.1099/00222615-13-1-57. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Cytotoxic activity of partially purified Escherichia coli alpha haemolysin. J Med Microbiol. 1982 Feb;15(1):11–21. doi: 10.1099/00222615-15-1-11. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte function in vitro. Infect Immun. 1982 Sep;37(3):966–974. doi: 10.1128/iai.37.3.966-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982 May;36(2):455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L., Warren J. W., Saah A. J., Caplan E. S., Tenney J. H., Hansen S., Granados J., Standiford H. C., Miller E. H., Jr A prospective randomized controlled trial of cefoxitin versus clindamycin-aminoglycoside in mixed anaerobic-aerobic infections. Surg Gynecol Obstet. 1982 May;154(5):715–720. [PubMed] [Google Scholar]
- Fried F. A., Wong R. J. Etiology of pyelonephritis: significance of hemolytic Escherichia coli. J Urol. 1970 Jun;103(6):718–721. doi: 10.1016/s0022-5347(17)62033-0. [DOI] [PubMed] [Google Scholar]
- Gadeberg O. V., Larsen S. O. In vitro cytotoxic effect of alpha-hemolytic Escherichia coli on human blood granulocytes. Correlation with size of alpha-hemolysin production. APMIS. 1988 Apr;96(4):337–341. [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983 Apr;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland I. B. Secretion of Escherichia coli haemolysin. Biochem Soc Trans. 1989 Apr;17(2):323–325. doi: 10.1042/bst0170323. [DOI] [PubMed] [Google Scholar]
- Hughes C., Hacker J., Roberts A., Goebel W. Hemolysin production as a virulence marker in symptomatic and asymptomatic urinary tract infections caused by Escherichia coli. Infect Immun. 1983 Feb;39(2):546–551. doi: 10.1128/iai.39.2.546-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Ripley M., Rochon Y., Pi-Jimenez E., Wright B. A role for hemolysin in Escherichia coli-induced inflammation in granulocytopenic rabbits. J Infect Dis. 1984 Dec;150(6):925–934. doi: 10.1093/infdis/150.6.925. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Lockatell C. V., Hall-Craigs M., Mobley H. L., Warren J. W. Uropathogenicity in rats and mice of Providencia stuartii from long-term catheterized patients. J Urol. 1987 Sep;138(3):632–635. doi: 10.1016/s0022-5347(17)43287-3. [DOI] [PubMed] [Google Scholar]
- Keane W. F., Welch R., Gekker G., Peterson P. K. Mechanism of Escherichia coli alpha-hemolysin-induced injury to isolated renal tubular cells. Am J Pathol. 1987 Feb;126(2):350–357. [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Jacobson S. H., Tullus K., Svenson S. B. P-fimbriae studies on the diagnosis and prevention of acute pyelonephritis. Infection. 1985 May-Jun;13(3):159–162. doi: 10.1007/BF01642881. [DOI] [PubMed] [Google Scholar]
- König B., König W., Scheffer J., Hacker J., Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986 Dec;54(3):886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVELL R., REES T. A. A filterable haemolysin from Escherichia coli. Nature. 1960 Nov 26;188:755–756. doi: 10.1038/188755b0. [DOI] [PubMed] [Google Scholar]
- Lomberg H., Hanson L. A., Jacobsson B., Jodal U., Leffler H., Edén C. S. Correlation of P blood group, vesicoureteral reflux, and bacterial attachment in patients with recurrent pyelonephritis. N Engl J Med. 1983 May 19;308(20):1189–1192. doi: 10.1056/NEJM198305193082003. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus D. M., Janis R. Localization of glycosphingolipids in human tissues by immunofluorescence. J Immunol. 1970 Jun;104(6):1530–1539. [PubMed] [Google Scholar]
- Marre R., Hacker J., Henkel W., Goebel W. Contribution of cloned virulence factors from uropathogenic Escherichia coli strains to nephropathogenicity in an experimental rat pyelonephritis model. Infect Immun. 1986 Dec;54(3):761–767. doi: 10.1128/iai.54.3.761-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Tenney J. H., Hull R. A., Warren J. W. Expression of type 1 fimbriae may be required for persistence of Escherichia coli in the catheterized urinary tract. J Clin Microbiol. 1987 Dec;25(12):2253–2257. doi: 10.1128/jcm.25.12.2253-2257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Low D., Romero I., Lark D., Vosti K., Falkow S., Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985 Aug 15;313(7):414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The toxic role of alpha-haemolysin in the pathogenesis of experimental Escherichia coli infection in mice. J Gen Microbiol. 1985 Feb;131(2):395–403. doi: 10.1099/00221287-131-2-395. [DOI] [PubMed] [Google Scholar]
- Trifillis A. L., Regec A. L., Trump B. F. Isolation, culture and characterization of human renal tubular cells. J Urol. 1985 Feb;133(2):324–329. doi: 10.1016/s0022-5347(17)48932-4. [DOI] [PubMed] [Google Scholar]
- Viano I., Eandi M., Santiano M. Toxic effects of some antibiotics on rabbit kidney cells. Int J Tissue React. 1983;5(2):181–186. [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., de Graaff J. Inactivation of haemolysin production in Escherichia coli by transposon insertion results in loss of virulence. Antonie Van Leeuwenhoek. 1983 Apr;49(1):23–30. doi: 10.1007/BF00457876. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Miller E. H., Jr, Fitzpatrick B., DiFranco D. E., Caplan E. S., Tenney J. H., Anthony W. C. A randomized, controlled trial of cefoperazone vs. cefamandole-tobramycin in the treatment of putative, severe infections with gram-negative bacilli. Rev Infect Dis. 1983 Mar-Apr;5 (Suppl 1):S173–S180. doi: 10.1093/clinids/5.supplement_1.s173. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Mobley H. L., Trifillis A. L. Internalization of Escherichia coli into human renal tubular epithelial cells. J Infect Dis. 1988 Jul;158(1):221–223. doi: 10.1093/infdis/158.1.221. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]