Abstract
Objectives
Human cord blood (CB) is a potential source of hematopoietic stem cells (HSC) for gene therapy to treat patients with hematopoietic disorders. However, limited numbers of CB CD34+ cells, low transduction efficiency with lentiviral vectors (LVs), and low engraftment efficiency of NOD/SCID repopulating cells (SRC), a measure of HSC, are blocks to this procedure. To optimize culture and transduction conditions, we compared various lengths of time for pre-stimulation before transduction, transduction duration, and post-transduction cell culture.
Materials and methods
We used a lentiviral vector to transduce human cord blood CD34+ cells followed by engraftment into NOD/SCID mice. We evaluated the effects of pre-stimulation and transduction time, and optimized the ex vivo cell culture duration before transplantation.
Results
We were able to achieve up to 40% transduction efficiency and up to 50% engraftment efficiency of SRC in CB CD34+ cells when CD34+ CB cells were either not pre-stimulated or pre-stimulated in 1% FBS medium for 1 hr, followed by 5 hr transduction and 3 days culture in a cocktail of growth factors after transduction. No apparent functional changes of CB CD34+ cells were noted under these conditions.
Conclusion
This gene transduction/cell expansion protocol is the first systematic study to optimize pre-stimulation time, transduction time and very importantly, ex vivo culture time after transduction, and may be of use for LV gene transduction in a gene therapy setting.
INTRODUCTION
One limitation for use of gene therapy as a treatment modality for genetic disorders and cancer has been low numbers of engrafted gene transduced donor cells in transplant recipients. This is an important impediment for successful gene therapy for humans and is also seen in gene transfer studies using large animals and primates 1–4. This limitation has been mainly attributed to: low efficiency gene transduction of long-term marrow repopulating hematopoietic stem cells (HSCs), and decreased efficiency of engraftment of transduced HSCs.
Cord blood (CB) is an established source of HSCs for allogeneic transplantatio2, 5. However, HSCs from CB are limited in numbers. Ex-vivo expansion of HSC has been used to compensate for the limiting numbers of CB HSCs 6–9, but several groups have suggested that ex-vivo expansion alters HSC function 10–12. Effectively transducing and expanding HSCs would be of practical importance. Lentiviral vectors have been used to transduce human CD34+ cells that engraft mice with non obese diabetes severe combined immunodeficiency (NOD/SCID) 13. We report a means to greatly enhance these activities for CB CD34+CD38− cells, NOD/SCID repopulating cells (SRCs), and hematopoietic progenitor cells (HPCs), using lentivirus vector (pCSCGW)-induced gene transduction.
MATERIALS AND METHODS
LV production
A third generation of replication-defective self-inactivating HIV-based lentiviral vector pCSCGW expressing EGFP under the control of an internal promoter-the immediate-early human cytomegalovirus, pseudotyped with vesicular stomatitis virus-G protein (VSV-G), was generated by transient co-transfection of with VSV-G-expressing construct pMD.G , rev-expressing construct pRSV.Rev, and packaging construct pMDL.RRE into 293T cells (ATCC, Manassas, VA) using lipofetamine 2000 transfection, according to the manufacturer's recommendations (Invitrogen, Carlsbad, CA). 293T cells were grown on collagen I-coated 100-mm dishes (BD Labware, Bedford, MA) in Dulbecco modified Eagle medium (DMEM; Biofluids, Rockville, MD) containing 10% heat-inactivated FBS (JRH Biosciences, Lenexa, KS). Culture medium was replaced with fresh medium before DNA delivery in 1% FBS IMDM. Cell culture supernatant was collected 24 hours after transfection, filtered through a 0.45-µm pore size low protein binding filter (Millipore, Carrigtwohill, Co. Cork, Ireland), aliquoted, and fast frozen in liquid nitrogen as LV stock for transduction.
LV transduction of human CB CD34+ cells
Human CB was collected and used according to institutional guidelines. CD34+ cells were purified from CB within 10 hours of CB collection. Cells obtained by immuno-magnetic selection (Miltenyi Biotec, Auburn, CA) were 94–97% CD34+. Isolated CD34+ cells were cultured in a flat bottom 24-well or 12-well plate in IMDM containing different concentrations of FBS. CB CD34+ cells were pre-stimulated with the following recombinant human cytokines: stem cell factor (SCF; 100 ng/ml), FLT3 ligand (100 ng/ml) and thrombopoietin (50 ng/ml) (SFT)14; purchased from R&D systems, Minneapolis, MN, for different lengths of time before LV transduction. For transduction, freshly purified CB CD34+ cells either not pre-stimulated or pre-stimulated with growth factors were incubated with LV viruses in the presence of SFT and 8 µg/ml polybrene. After transduction, cells were washed extensively and cultured in the IMDM medium. The cells were transduced at M.O.I from 10 to 50. Transduction efficiencies were assessed at different time points by determining the percentage of EGFP positive cells using FACS analyses. Cells were counted using a Z2 Coulter Particle Count and Size Analyzer (Beckman Coulter, Fullerton, CA).
In vitro hematopoietic colony-forming cell (CFC) assay
LV-transduced or mock transduced human CB CD34+ cells were seeded in triplicate onto a 35mm dish at a density of 250 cells per dish in 1.0 ml of 1% methylcellulose culture medium containing 100 mM 2-mercaptoethanl, 2 mM L-glutamine, 30% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT). Recombinant human erythropoietin (1 U/ml), SCF (50 ng/ml), human interleukin-3 (IL-3; 10 ng/ml), and granulocyte macrophage colony stimulating factor (GM-CSF; 10ng/ml) were included in the culture medium to stimulate colonies from multipotential (CFU-GEMM), erythroid (BFU-E; burst-forming units-erythroid), and granulocyte macrophase (CFU-GM) progenitor cells. Epo was purchased from Amgen Inc. (Thousand Oaks, CA). IL-3 and GM-CSF, were purchased from R & D Systems. Colonies were scored after 14 days incubation at 5% CO2 and lowered (5%) O2 tension.
Cell staining analysis
Mouse blood samples were treated with red blood cell lysis buffer (0.16 ml NH4Cl, 0.1 M KHCO3, 0.1 mM EDTA) at room temperature for 5 min. Bone marrow cells of transplanted mice and treated blood cells were stained with human CD45 (hCD45)-allophycocyanin (APC). LV-transduced or mock transduced CB CD34+ cells were stained with hCD34-APC and hCD38 phycoerythrin (PE) (Miltenyibiotec, Germany) at dilutions according to manufacturer’s instructions. LV-transduced CB CD34+ cells and human cells engrafted into mouse bone marrow were examined for GFP expression using flow cytometry. About 100,000 events for each sample were acquired and analyzed on a FACSCalibur, using CellQuest software package (BD Biosciences Immunocytometry System, San Diego).
PCR analysis
Genomic DNA for PCR analysis was extracted from total CB CD34+ cells after LV transduction of human cells at 1 hr, 2.5 hrs, 5 hrs and 16 hrs, and from the same source of mock transduced CB CD34+, which served as a negative control, or from total NOD/SCID mice bone marrow cells transplanted with LV transduced human CB CD34+ cells, by using Promega Genomic DNA extraction kit following manufacturer’s instructions. When genomic DNA was extracted at early points, the kit picked up DNA that included a majority of pro-viral DNA, as well as LV integrated DNA. DNA analysis was performed by quantitative PCR. One to 20 ng of purified DNA was amplified for the specific EGFP fragment. The primers for the EGFP within LV were forward primer 5’-gccacaagttcagcgtgtccggcg3’ and reverse primer 5’ggcggacttgaagaagtcgtgctg3’. PCR was performed on a PE Thermocycler 9700 (PE Applied Biosystem, Foster City, CA) with a program of 94°C for 2 min, followed by 25 cycles of 94°C for 30 sec, 62°C for 30 sec and 72°C for 30 sec and one cycle of 72°C for 7 min. Human glyceraldehyde-3-phosphate dehydrogenase gene (GADPH) was included in the PCR as an internal control with GADPH-specific primers which span intron and exon, the forward primer 5’-ggactggctttcccataatttc3’ and reverse primer 5’-aggtcaggtccaccactgacacg3’. The sizes of PCR products for EGFP and GADPH were 100 bp and 306 bp respectively and amplified within a linear range. PCR products were analyzed in 1.2% agarose gel.
Transplantation of human CB CD34+ cells
Following in vitro culturing, LV-transduced or mock transduced CB CD34+ cells were washed, re-suspended in 0.5 ml PBS, and injected into 8 to 10 week old sub-lethally irradiated (300 cGy) NOD/SCID or NOD/SCID IL-2 receptor null (NS2)15, 16 mice via tail vein. 8 × 104 cells were injected per mouse for NOD/SCID mouse and 5 × 104 cells were injected per mouse for NS2 NOD/SCID mouse. All the animal procedures were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine. Mouse bone marrow was harvested from 8–20 weeks after transplantation for staining of human CD45+, followed by analysis for human CD45+ cells as well as GFP expression using flow cytometry.
Statistical analysis
Values are expressed as mean ± SD. Comparisons among groups were made using Student’s t test.
RESULTS
Growth and LV transduction of CD34+ CB cells in different serum concentrations
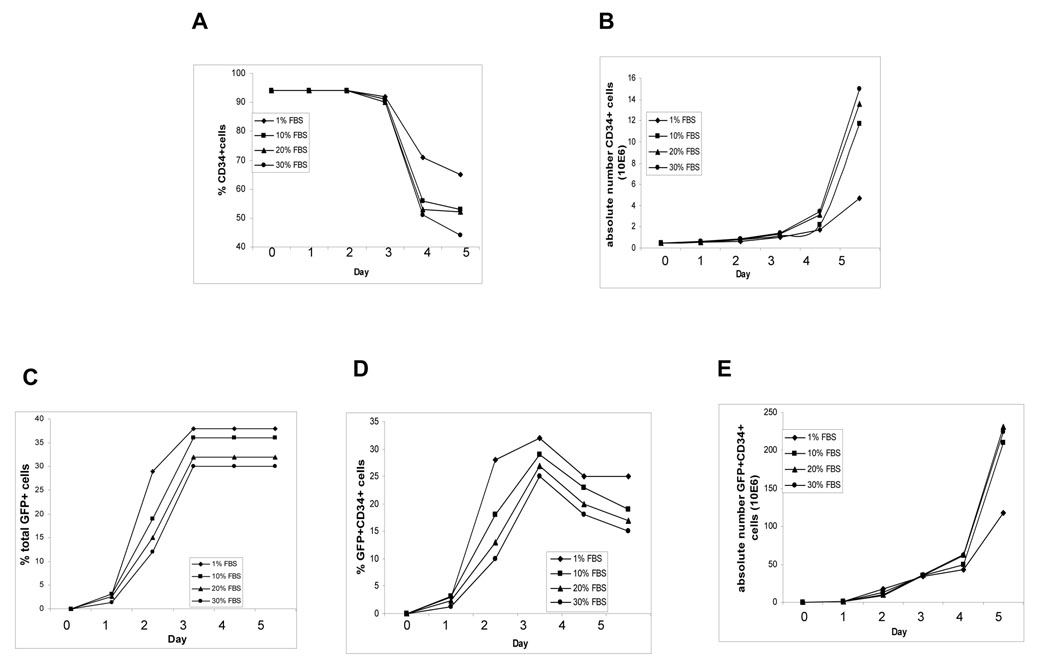
Unlike cell lines, there are no standardized recipes for culturing CB CD34+ cells. We used IMDM containing FBS plus 50 ng/ml Tpo, 100 ng/ml FL, and 100 ng/ml SCF to culture cells. To obtain high LV transduction efficiency with minimal changes in cell function, we evaluated effects of FBS concentration in culture medium on LV transduction efficiency of CB CD34+ cells, as well as on proliferation, differentiation, and colony forming ability of these cells. CB CD34+ cells were transduced with LV for 5 hr in 1% FBS and then transduced cells were cultured in culture medium containing 1, 10, 20, or 30% FBS. The % CD34+ cells was similar from 1–3 days in the different serum concentration with the least decrease from 4–5 days at 1% FBS (Fig. 1A). Absolute numbers of CD34+ cells reached log phase growth between days 4 and 5 with the greatest increases in cell numbers at 10–30% FBS (Fig. 1B).
Fig. 1. Transduction of CB CD34+ cells with LVs.
Freshly isolated CB CD34+ cells were transduced with LVs for 5 hr. After extensive washing with PBS, the cells were cultured in IMDM containing SFT (100 ng/ml SCF, 100 ng/ml FL and 50 ng/ml Tpo) and FBS at 1, 10, 20, or 30%. Cells were counted and monitored daily for GFP expression for transduction efficiency daily. Cells were also stained daily with anti-CD34 antibody, and FACS was used to determine the percentage and numbers of CD34+ cells. A) % CD34+ cells, B) absolute number of CD34 + cells, C) % GFP+ cells, D) % GFP+CD34+ cells, and E) absolute number of GFP+CD34+ cells.
The percent of GFP+ cells was maximal by day 3 (Fig. 1C). At day 3, medium containing 1, 10, 20, and 30% FBS gave rise to 38, 35, 32, and 28% GFP+ cells, respectively, with no further changes through day 5. The % GFP+CD34+ cell population was maximal at day 3 in all four FBS concentrations, with the highest percentages of GFP+CD34+ cells detected in 1% FBS (Fig. 1D). There were no differences in absolute numbers of GFP+CD34+ cells among all 4 serum groups during the first 4 days (Fig. 1E). Although the absolute numbers of GFP+CD34+ cells was greatest by day 5, the size of colonies derived from the CD34+ population of colony forming cells was smaller by days 4–5, especially in cells cultured in the higher FBS concentration (data not shown). We interpret this as HPC function decreases if the ex vivo culture exceeds 3 days. Ex-vivo culture of LV-transduced CB CD34+ cells in 1% FBS culture medium for up to 3 days most consistently facilitated expression of the gene of interest without adverse effects on proliferative capacity of HPCs.
Virus loading reaches saturation with 5 hours virus exposure time
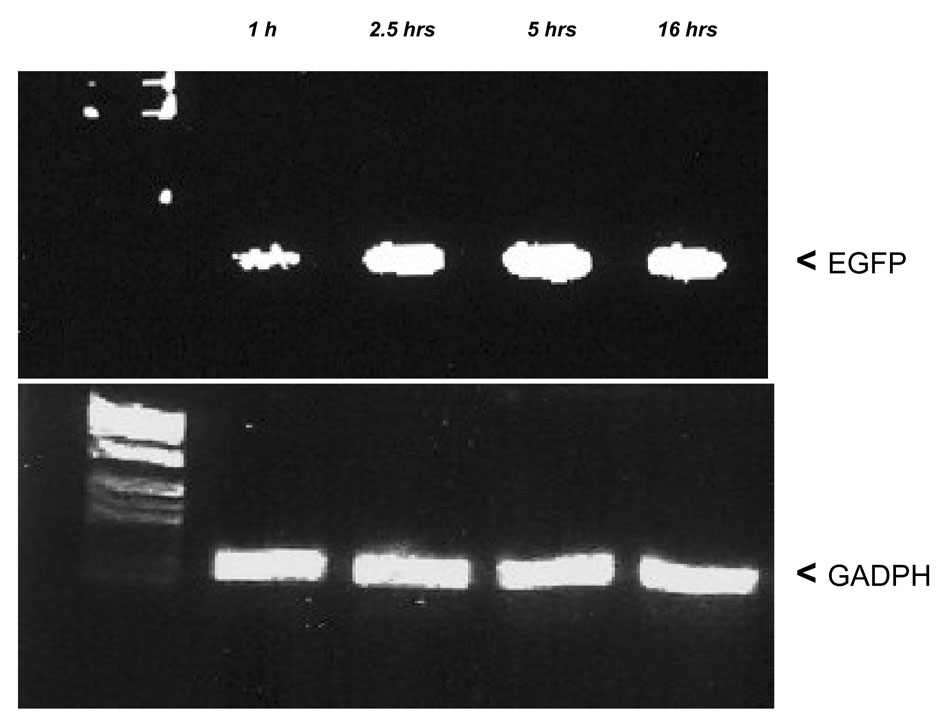
HIV-1 based lentiviral vector stable integration into the genome of the infected cell involves reverse transcription of viral RNA to yield a linear double-stranded DNA copy and then integration of this viral cDNA into a chromosome of the host cell 17. To test how long it takes cells exposed to lentivirus to reach virus saturation, CD34+ cells were purified, and lentivirus supernatant was added to the cells for 1, 2.5, 5 and 16 hours. Cells were then washed with PBS and extracted for whole genomic DNA. The total virus loaded onto the cells was compared by amplifying the GFP fragment on the vector. Virus loading reached a peak at 5 hours (Fig. 2). Longer exposure times might not a good choice because of envelope toxicity to the cells.
Fig. 2. Detection of total CD34+ genomic EGFP DNA by quantitative PCR.
After CD34+ cells were exposed to virus for 1, 2.5, 5 and 16 hours, cells were extensively washed. Total CD34+ cell genomic DNA was extracted. EGFP was amplified, and GADPH was amplified as an internal control. This is one of 3 representative experiments.
CFC of LV-transduced CB CD34+ cells with continuing culture in vitro
After 5 hours of exposing freshly isolated CB CD34+ cells to virus, the gene of the transduced virus starts to express and reaches maximum after 3 days of culture with growth factors (SFT) (Figure 1D). To determine the best time point for colony-forming cell (CFC) assay, transduced cells were put in semi-solid culture with growth factors immediately after 5 hour transduction at day 0 or transduced cells were left in suspension culture with SFT for an additional 3 days (total time of 72 hrs from CD34+ cells isolation) prior to plating cells in semi-solid culture with growth factors. As a control, the same cell culture conditions were used which included the same medium and concentration of growth factors and polyberene, except control medium was added instead of the virus. At day 3, the cell number for both groups were 2 times greater compared to that at day 0 because of the cell proliferation. However, we calculated the cell numbers to plate based on the pre-expansion cell numbers. Table 1 presents results from one of the two representative experiments which show the same trend. GFP positive (CFU-GM, BFU-E, and CFU-mix) colonies were assessed. The percentage of GFP+ colonies increased from ~7.5% at day 0 to 37~60 % at day 3. This demonstrates that cell culture with SFT after a short transduction period without pre-stimulation is most effective in transduction/expression of HPC.
Table 1.
Continued in vitro culture is required for efficient gene transduced colony expression of GFP+ cells after short term transduction of CB CD34+ cells without pre-stimulation#
| Total Colony Number | Number (%*) E-GFP+ Colonies | ||||
|---|---|---|---|---|---|
| Transduction | Days of culture after transduction | CFU-GM | BFU-E+CFU-GEMM | CFU-GM | BFU-E +CFU-GEMM |
| − | D0 | 23 ± 2.5 | 16 ± 3.0 | - | - |
| + | D0 | 22 ± 4.7 | 17 ± 3.2 | 2 ± 0.6 (7.5%) | 1 ± 0.6( 7.5%) |
| − | D3 | 22 ± 2.8 | 9 ± 2.1 | - | - |
| + | D3 | 27 ± 3.1 | 14 ± 1.5 | 10 ± 1.0 (37%) | 9 ± 1.1 (60%) |
Freshly isolated CB CD34+ cells were transduced with LV for 5 hours without pre-stimulation. After extensive washes with PBS, cells were cultured in IMDM medium and growth factors (SFT) for 0 hour (D0) or for 72 hours (including the transduction time) (D3); input cell number is 250/ml; CFU-GM, GEMM/BFU-E and GFP+ colonies were counted 14 days afterward.Resrults are expressed as mean +/− SD.
The percentage of GFP+ colonies out of the total colonies counted.
This is one of 2 representative experiments.
Effects of pre-stimulation and transduction duration on the transduction efficiency and differentiation of CB CD34+ cells
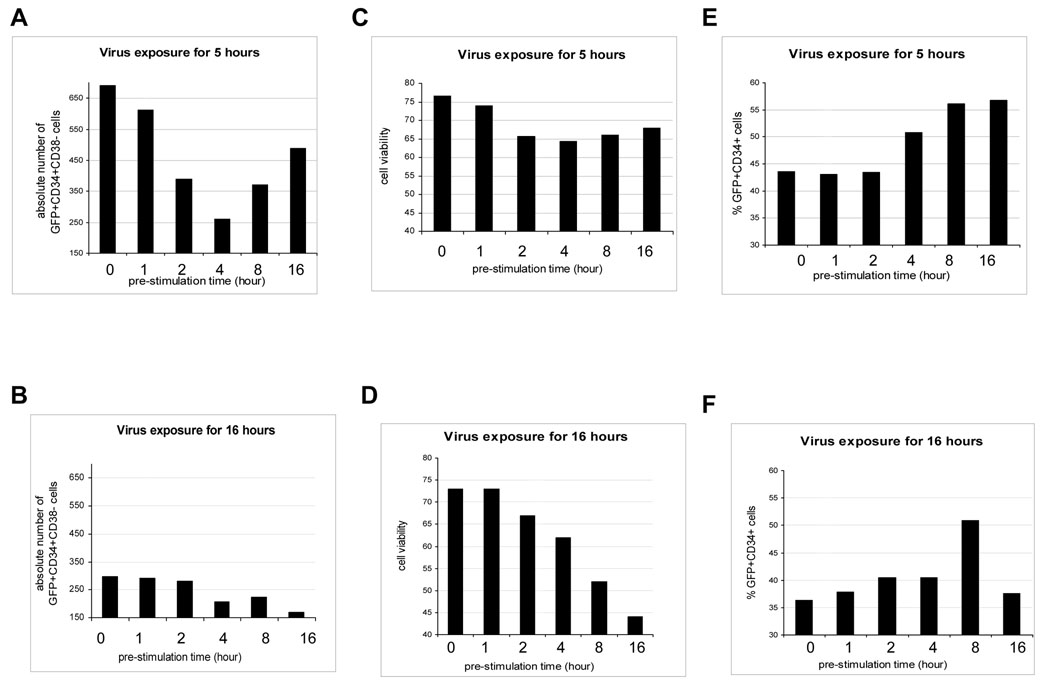
We next evaluated conditions of pre-stimulation and virus exposure time of CB CD34+ cells for the highest transduction efficiency of CD34+ cells, and also for the more immature subset of CD34+CD38 − cells. To this end, we cultured freshly isolated CB CD34+ cells in medium containing 1% FBS and growth factors (SFT) for 0, 1, 2, 4, 8 and 16 hr, transduced these cells with LV for 5 or 16 hr, washed the cells, and continued in vitro culture in supernatant with SFT for 3 days. Pre-stimulation before LV transduction resulted in no significant changes in the percentage of viable CD34+ cells (data not shown). Cells without pre-stimulation and virus exposure for 5 hours gave rise to the greatest number of GFP+CD34+CD38− cells (Figure 3A compared to 3B). Deceased numbers of GFP+CD34+CD38− cells were noted with virus exposure for 16 hrs, compared to those with virus exposure for 5 hrs. The highest cell viability was found in the groups without pre-stimulation or with 1 hr pre-stimulation (Figure 3C and 3D). Thus, optimal numbers of transduced CD34C+D38− cells were found with no pre-stimulation and a 5 hr virus exposure time prior to 3 days of culture in SFT (Figure 3A), even though pre-stimulation can increase the percentage of gene transduced CD34+ cells (Figure 3E and 3F).
Fig. 3. Effects of different pre-stimulation times vs. LV exposure period for freshly isolated CB CD34+ cells.
Freshly isolated CB CD34+ cells were pre-stimulated for 0, 1, 2, 4, 8 and 16 hours individually with cell growth factors (SFT), and cells were analyzed at 72 hours after transduction. Absolute numbers of GFP+CD34+CD38− cells after exposure to virus for 5 (A) or 16 hours (B) were determined by scoring CD38− cells in the gated GFP+CD34+ cell population. Cell viability, after cells were exposed to virus for 5 (C) or 16 (D) hours, was determined by FACS. Transduction efficiency of CD34+ cells exposed for 5 (E) or 16 hours (F) was assessed by determining the percentage of GFP+ as well as CD34+ cells by FACS analysis.
Engraftment of LV transduced CB CD34+ into NOD/SCID mice
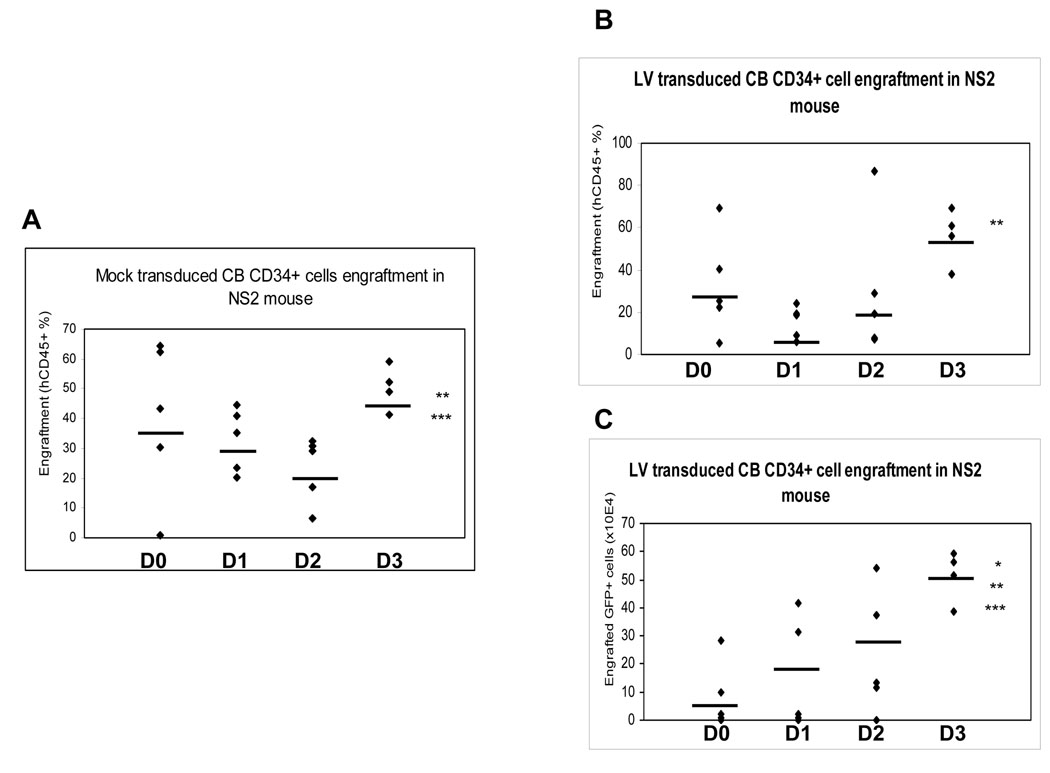
The in vitro HPC data above suggested that no pre-stimulation phase, a 5 hr virus exposure time and 3 days of culture in 1% FBS plus SFT resulted in a higher percentage of gene marked HPC as determined by colony assay of CD34+ cells and phenotypic analysis of CD34+CD38− cells. Since human SRC are found in the CD34+CD38− population of cells, we evaluated these optimal conditions for gene transduction of SRC. For a preliminary study, we used NOD/SCID mice as a model for transplantation. Limited numbers (8×104) of total transduced CB CD34+ cells were transplanted into sub-lethally irradiated NOD/SCID mice with two groups compared. The first group was transplanted immediately after 5 hrs transduction of CD34+ cells. In this case, we were not able to detect GFP+ expression even though human cell engraftment was successful. The second group was transplanted after 5 hr transduction and continued culture with SFT for 2 more days. In this group, we detected about 18% of GFP+ cells within the human CD45+ cell population in BM (data not shown). This demonstrated that continued culture with SFT after a short period of transduction is important. These results led us to study the optimum duration for continued culture time using NS2 NOD/SCID mice as recipients. NS2 NOD/SCID mice are superior recipients for xeno-transplantation compared with NOD/SCID mice 15, 16. Freshly isolated CB CD34+ cells were transduced with LV for 5 hr without a pre-stimulation phase, and these cells cultured in 1% FBS medium containing SFT for 0, 1, 2 or 3 days. Cells were then transplanted into sub-lethally irradiated NS2 NOD/SCID mice. Numbers of cells injected into NS2 NOD/SCID mice after 1–3 days were equivalent to whatever was present based on the initiation cell count of 5 × 104 starting CD34+ cells. Mock-transduced CB CD34+ cells were included as a control. Eight weeks after transplantation, bone marrow was harvested from both femurs of these mice and analyzed for hCD45+ expression and GFP+ expression. In the mock transduction group, the average engraftment of hCD45+ cells that were cultured for 0, 1, 2, and 3 days was respectively: 41 ± 26.1%, 33 ± 10.6%, 23 ± 11.1%, and 49 ± 7.6%, respectively (Figure 4A). In comparison, engraftment of hCD45+ cells that were cultured for 0, 1, 2 and 3 days in the LV transduction group was 33 ± 23.9%, 15 ± 7.6%, 37 ± 30.6% and 56 ± 13.3%, respectively (Figure 4B). In both mock and LV transduction groups the engraftment efficiency was highest for the cells that were cultured for 3 days compared to those cultured for 0, 1, or 2 days. More importantly, the absolute number of hGFP+CD45+ cells in mouse bone marrow (expressed × 104 ) were 6.8 ± 12.3 for 0 day culture, 14.6 ± 20.3 for 1 day culture, 23.4 ± 21.8 for 2 day culture, and 51.4 ± 9.1 for 3 day culture (Figure 4C). Thus, the greatest numbers of GFP+CD45+ cells were found in NS2 NOD/SCID mice transplanted with CD34+ CB cells that were cultured for 3 days after virus contact; this corroborated the in vitro results which demonstrated that extended in vitro culture post-transduction is important for successful transduction of CD34+ cells and HPC.
Fig. 4. Engraftment of CB CD34+ cells into NS2 NOD/SCID mice.
Freshly isolated CD34+ cells were mock transduced (A) or transduced with LVs (B & C), washed, and cultured in IMDM containing SFT (100 ng/ml SCF, 100 ng/ml FL and 50 ng/ml Tpo) and 1% FBS for 0, 1, 2, or 3 days. Cells were injected into sub-lethally irradiated NS2 NOD/SCID mice via tail vein. Bone marrow from these mice was harvested 8 weeks after injection and stained for surface expression of human (h) CD45 using anti-hCD45 antibody. hCD45+ and GFP+ cells were analyzed using FACS. **P<0.01 for day 2 versus day 3, ***P<0.05 for day 1 versus day 3 (Fig. 4A); **P<0.01 for day 1 versus day 3 (Fig. 4B); *P<0.0005 for day 0 versus day 3; **P<0.01 for day 1 versus day 3; ***P<0.05 for day 2 versus day 3 (Fig. 4C).
LV gene becomes silent without continuing culture in vitro for engraftment of NOD/SCID mice
The above engraftment data suggested that the LV was expressed at the highest level in NS2 NOD/SCID mice transplanted with CD34+ CB cells that were cultured for 3 days after virus contact (Figure 4C). Since we transduced the cells with LV for the same time period, the difference in expression was a result of the in vitro culture time. To evaluate whether the very low LV expression in NS2 NOD/SCID mice engrafted with cells in which there was no further culture time after virus contact resulted from transduced cells that were not able to engraft or transduced cells that could engraft but became silent in vivo, we amplified EGFP by genomic DNA PCR from bone marrow cells of NS2 NOD/SCID mice at 8 weeks which were transplanted with CD34+ CB cells that were cultured for 0 or 3 days after virus contact (Figure 5A). The human GADPH gene was amplified by genomic DNA PCR first to normalize the human CD45+ DNA amount. The results showed that the LV integrated DNA increased about 30% with 3 days culture in vitro compared with no additional culture in vitro (Figure 5A). However, the EGFP expression from the flow cytometry analysis showed 68% increase with 3 days culture in vitro compared with no additional culture in vitro (Figure 5B). This suggests that part of the LV integration becomes silent without additional culture in vitro. This discrepancy between LV integration and LV expression also suggested that there could be other mechanisms involved in addition to gene silencing.
Fig. 5. LV gene silencing without additional culture in vitro.
Freshly isolated CD34+ cells were transduced with LVs for 5 hours, washed and injected into sub-lethally irradiated NS2 NOD/SCID mice via tail vein (mice numbered 1,2 and 3) or injected into the mice after continued culture in vitro for an additional 3 days (mice numbered 4, 5, and 6). Bone marrow from these mice was harvested 8 weeks after injection. Part of the bone marrow cells were extracted for genomic DNA (A) and the rest of the cells were analyzed for GFP+ cells (B). Human specific GADPH gene was amplified in a linear range to normalize equal human CD45+ DNA amount. DNA from the same strain without engraftment was used as a negative control. Mock transduction engraftment was used as a negative control. Rel equals relative expression level.
Longer term studies of human cell engraftment and EGFP expression in vivo
The experiments in Figure 4 with NS2 NOD/SCID mice transplanted with LV transduced CD34+ CB cells showed successful engraftment in BM at 8 weeks. To test whether our transduction protocol sustains stable LV gene expression in vivo over longer term periods, we transduced human CB CD34+ cells and transplanted them into the NS2 NOD/SCID mice, the same way as noted above for Figure 4. We followed the % hCD45+ cells (Figure 6A) and the % of EGFP+ cells in the hCD45+ cell populations (Figure 6B) in the peripheral blood (PB) of these mice. At 8 weeks post-transplant, about 8 percent of hCD45 + cells were detected; 19% within the hCD45+ cell population were EGFP positive (Figure 5A and 5B). Both the hCD45+ cell percentage and percentage of EGFP+ within the hCD45+ cells in PB were much lower than in bone marrow at this time point (Figure 6C). When we continued monitoring the hCD45+ cell percentage as well as the percentage of EGFP+ cells within the hCD45+ cells in PB, we found that the hCD45+ percentage reached 19 percent and EGFP+ cell percent within the hCD45+ cells reached to 31 percent at 16 weeks; the hCD45+ percent in PB continued to go up to 24 percent and EGFP+ cell percent within hCD45+ cells went up to 42 percent at 20 weeks and this level was maintained at 28 weeks (Figure 6A and 6B). The intensity of EGFP transgene expression in PB still remained high after engraftment at 28 weeks. This suggests maintenance of the long-term repopulating potential of LV transduced cells using our short term transduction protocol.
Fig. 6. Expression of LV transduced SCID Repopulating cells is sustained during long-term engraftment.
LV-transduced human CD34+ cells were transplanted into NOD/SCID NS2 mice. Peripheral blood was assessed at 8, 16, 20 and 28 weeks from the mouse tail vein (n = 20) or at 8 weeks from bone marrow (n = 10). After lysis of red blood cells, hCD45+ antibody was applied for staining followed by analysis by flow cytometry. hCD45+ percentage (A) and EGFP positive percentage within the hCD45+ population (B) within peripheral blood or hCD45+ percentage as well as EGFP positive percentage within the hCD45+ population at 8 weeks in bone marrow (C) was monitored.
DISCUSSION
Freshly isolated human CB CD34+CD38− cells are mostly in a quiescent stage (G0) and the percentage of these G0 cells decreases as they gain CD38 expression 18, 19. In this study, we have found that the highest number of LV transduced human CD34+ and CD34+ CD38− cells were obtained when the freshly isolated CD34+ cells were immediately exposed to LV for 5 hr without pre-stimulation, and then cultured for 3 more days in vitro before being transplanted into NOD/SCID mice. Even though LV is capable of transducing CD34+ cells at a higher percentage following pre-stimulation (Figure 3E and 3F), our results showed that the percentage (data not shown) as well as the absolute number of the LV-transduced CD34+CD38− cells increased in the LV-tranduced CD34+ cells with no pre-stimulation compared to that with pre-stimulation (Figure 3A and 3B). Thus, if CD34+ are pre-stimulated, the quiescent CD34+ cells begin to move into G2 and S phases of the cell cycle, so that CD34+ cells gain CD38 expression resulting in decreases in CD34+CD38− cells. Studies have shown that LV transiently arrests transduced cells at a high virus titre 20, and transduction is associated with a marked decrease in cell growth rate 21, which suggests that transduction itself might delay the transduced cells from gaining CD38 expression. Slowing down the cycling of CD34+ cells in the quiescent stage (G0) from moving into an active cell cycle phase (G2 and S) without the pre-stimulation, plus the transduction process itself may be part of the reasons for maintaining the high percentage of transduced CD34+CD38− cells.
It has been reported that Rhesus bone marrow CD34+ cells transduced with Moloney-type retrovirus for 4 days and continued in culture for an additional two days with CH-296 and SCF gave good engraftment 22. Also, human BM CD34+ cells transduced with Moloney-type retrovirus for 2 consecutive days with 1 day pre-stimulation with CH-296 and SCF gave a better engraftment than without the extended 2 days culture 11. These studies suggested the importance of extended culture time following retrovirus transduction. Our present studies have significantly extended these observations to enhancement of LV-transduced cord blood CD34+CD38− SRC. Since LVs have advantages over RVs in that LVs do not need a pre-stimulation phase, we used LVs to transduce human CB CD34+ cells for 5 hrs with no pre-stimulation. Thus, cells were in culture less time through the transduction phase allowing us to better compare the optimum additional culture time before CD34+ cells experience functional change and decreased HPC and SRC activity.
Our data showed that CB CD34+ cells that were transduced with LV for 5 hr without pre-stimulation and subsequently subjected to additional culturing in vitro gave rise to high engraftment efficiency of gene transduced cells. Less than 3 days in vitro culturing of cells following LV tranduction resulted in a much lower percentage of gene transduced cells in engrafted mice than with 3 days additional culture. Freshly isolated CB CD34+ cells are likely still at the G0/G1 phase when they receive no pre-stimulation and 5 hr transduction. LVs such as human immunodeficiency virus type 1 (HIV-1) can readily enter quiescent T cells in culture and synthesize partially reverse-transcribed viral DNA or in full-length, but transcription of intergrated viral DNA and the productive infection depends on subsequent cell activation and division 17, 23–25. Our data also show that LV DNA integration is much greater with additional culture (up to 72 hours) after short term transduction, than without additional culture time for the long term transplantation study (Figure 5A). This suggests that when HIV-based LVs transduce freshly isolated CB CD34+ cells for a short period, the transduced cells most likely still stay in a quiescent state. If they are transplanted at this stage, this population will remain quiescent in vivo for a while, and the transduced LVs likely remain silent to some degree. Our data demonstrate that the gene of interest after LV transduction expressed at maximum with 3 days culture after a short transduction time period without pre-stimulation in vitro (Figure 1). Our results suggest that the cells should be transplanted when the gene of interest from LV transduction first reaches the highest level of expression. In this context, the cell that has the gene of interest expressed stably before injection in vivo likely minimizes the opportunity for gene silencing maximizing gene integration. If CD34+ cells experience a long pre-stimulation phase, e.g., overnight or two days before LV transduction, the cells have already entered G2 or S phases. Even though the transduction percentage is higher, the transduced CD34+CD38− cell population is decreased and cell viability is decreased as we have shown. Since SRC are found in CD34+CD38− population 19, we regard the percentage as well as the absolute number of gene transduced CD34+CD38− cells to be more important than the percentage of gene transduced CD34+ itself in the context of engraftment of gene transduced cells.
There is a great variation among different research groups regarding the optimal virus exposure period 8, 18, 26–29. We tested different virus exposure times of 1, 2.5, 5 and 16 hr. We found that 5 hr virus exposure time was sufficient for transduction, and longer virus exposure periods did not show further increases either in virus loading into the cells or in transduction efficiency. Longer virus exposure periods could adversely affect the cells in two ways even though the transduction efficiency remains quite similar. One is that cell viability decreases (Figure 3C and 3D), which could be explained by VSV-G envelope toxicity to the transduced cells 30, 31. The other is a decrease in the GFP+ CD34+CD38− population, which might relate to the cell cycle changes discussed above.
In summary, we have established an efficient protocol to culture and transduce CB CD34+CD38− cells. Three critical parameters were optimized in this protocol to achieve the highest transduction and engraftment efficiency of these cells: (1) no or very short pre-stimulation of CB CD34+ cells in 1% FBS medium before LV transduction, (2) transduction with LV for 5 hr with short or no pre-stimulation phase, and (3) ex-vivo culture of cells for 3 days in 1% FBS medium with growth factors (SFT) post-transduction. The advantage of the this protocol is that only 5 hours transduction time is applied instead of a traditional 16 hours or even longer time, which greatly improved CB CD34+ cell viability. This is to our knowledge the first systematic study to optimize the pre-stimulation time, transduction time and very importantly, the ex vivo culture time after transduction. We believe this enhanced efficiency of LV transduction of engrafting SRC (HSCs), as well of hematopoietic progenitor cells (CFCs), has practical relevance for human gene therapy protocols. While we found low serum levels optimum, the protocol will have to be evaluated with appropriate clinical grade material prior to being considered for actual gene therapy in a human clinical trial setting.
ACKNOWLEDGEMENT
We would like to thank Christopher Touloukian M.D, (Indiana University School of Medicine) for providing PCSCGW, pMD.G, pRSV.Rev and pMD.L plasmids.
We thank Mary Dinauer, M.D. PhD, and Mervin Yoder, M.D, Department of Pediatrics, Indiana University School of Medicine, for reading the final drafts of the manuscript and for their helpful suggestions.
These studies were supported by the following US Public Health Service Grants to HEB: a project in NIH P01 HL 53586, NIH R01 HL 67384 and NIH R01 HL56416 to HEB. TC is supported by NIH T32 training grant DK07519 to HEB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Brenner MK, Heslop HE. Is retroviral gene marking too dangerous to use? Cytotherapy. 2003;5:190–193. doi: 10.1080/14653240310001307. [DOI] [PubMed] [Google Scholar]
- 2.Broxmeyer HE, Smith FO. Chapter 39. In: Applelbaum FR, Forman SJ, Negrin S, Blume KG, editors. Cord Blood Hematopoitic Cell Transplantation. 4th Edition. Cambridge, MA: Blackwell Scientific Publications; 2008. [Google Scholar]
- 3.Dando JS, Aiuti A, Deola S, et al. Optimisation of retroviral supernatant production conditions for the genetic modification of human CD34+ cells. J Gene Med. 2001;3:219–227. doi: 10.1002/1521-2254(200105/06)3:3<219::AID-JGM184>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Emery DW, Nishino T, Murata K, et al. Hematopoietic stem cell gene therapy. Int J Hematol. 2002;75:228–236. doi: 10.1007/BF02982035. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V, Boyer-Chammard A, et al. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 6.Ando K, Yahata T, Sato T, et al. Direct evidence for ex vivo expansion of human hematopoietic stem cells. Blood. 2006;107:3371–3377. doi: 10.1182/blood-2005-08-3108. [DOI] [PubMed] [Google Scholar]
- 7.Bhatiaz M, Bonnet D, Kapp U, et al. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997;186:619–624. doi: 10.1084/jem.186.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piacibello W, Bruno S, Sanavio F, et al. Lentiviral gene transfer and ex vivo expansion of human primitive stem cells capable of primary, secondary, and tertiary multilineage repopulation in NOD/SCID mice. Nonobese diabetic/severe combined immunodeficient. Blood. 2002;100:4391–4400. doi: 10.1182/blood.V100.13.4391. [DOI] [PubMed] [Google Scholar]
- 9.Ueda T, Tsuji K, Yoshino H, et al. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest. 2000;105:1013–1021. doi: 10.1172/JCI8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorrell C, Gan OI, Pereira DS, et al. Expansion of human cord blood CD34(+)CD38(−) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000;95:102–110. [PubMed] [Google Scholar]
- 11.Gothot A, van der Loo JC, Clapp DW, et al. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34(+) cells in non-obese diabetic/severe combined immune-deficient mice. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- 12.Rebel VI, Tanaka M, Lee JS, et al. One-day ex vivo culture allows effective gene transfer into human nonobese diabetic/severe combined immune-deficient repopulating cells using high-titer vesicular stomatitis virus G protein pseudotyped retrovirus. Blood. 1999;93:2217–2224. [PubMed] [Google Scholar]
- 13.Miyoshi H, Smith KA, Mosier DE, et al. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Ray NT, Atkinson SJ, et al. Protein phosphatase 2A plays an important role in stromal cell-derived factor-1/CXC chemokine ligand 12-mediated migration and adhesion of CD34+ cells. J Immunol. 2007;179:3075–3085. doi: 10.4049/jimmunol.179.5.3075. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 17.Zack JA, Haislip AM, Krogstad P, et al. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazurier F, Gan OI, McKenzie JL, et al. Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and culture-induced stem cell impairment. Blood. 2004;103:545–552. doi: 10.1182/blood-2003-05-1558. [DOI] [PubMed] [Google Scholar]
- 19.McKenzie JL, Gan OI, Doedens M, et al. Reversible cell surface expression of CD38 on CD34-positive human hematopoietic repopulating cells. Exp Hematol. 2007;35:1429–1436. doi: 10.1016/j.exphem.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Lee CI, Kohn DB, Ekert JE, et al. Morphological analysis and lentiviral transduction of fetal monkey bone marrow-derived mesenchymal stem cells. Mol Ther. 2004;9:112–123. doi: 10.1016/j.ymthe.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Nayak SK, McCallister T, Han LJ, et al. Transduction of human renal carcinoma cells with human gamma-interferon gene via retroviral vector. Cancer Gene Ther. 1996;3:143–150. [PubMed] [Google Scholar]
- 22.Takatoku M, Sellers S, Agricola BA, et al. Avoidance of stimulation improves engraftment of cultured and retrovirally transduced hematopoietic cells in primates. J Clin Invest. 2001;108:447–455. doi: 10.1172/JCI12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiser J, Harmison G, Kluepfel-Stahl S, et al. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci U S A. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spina CA, Guatelli JC, Richman DD. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zack JA, Arrigo SJ, Weitsman SR, et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Golob J, Kelleher E, et al. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood. 2002;99:399–408. doi: 10.1182/blood.v99.2.399. [DOI] [PubMed] [Google Scholar]
- 27.Evans JT, Cravens P, Gatlin J, et al. Pre-clinical evaluation of an in vitro selection protocol for the enrichment of transduced CD34+ cell-derived human dendritic cells. Gene Ther. 2001;8:1427–1435. doi: 10.1038/sj.gt.3301530. [DOI] [PubMed] [Google Scholar]
- 28.Woods NB, Fahlman C, Mikkola H, et al. Lentiviral gene transfer into primary and secondary NOD/SCID repopulating cells. Blood. 2000;96:3725–3733. [PubMed] [Google Scholar]
- 29.Zhang S, Joseph G, Pollok K, et al. G2 cell cycle arrest and cyclophilin A in lentiviral gene transfer. Mol Ther. 2006;14:546–554. doi: 10.1016/j.ymthe.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Di Nunzio F, Piovani B, Cosset FL, et al. Transduction of human hematopoietic stem cells by lentiviral vectors pseudotyped with the RD114-TR chimeric envelope glycoprotein. Hum Gene Ther. 2007;18:811–820. doi: 10.1089/hum.2006.138. [DOI] [PubMed] [Google Scholar]
- 31.Qiao J, Moreno J, Sanchez-Perez L, et al. VSV-G pseudotyped, MuLV-based, semi-replication-competent retrovirus for cancer treatment. Gene Ther. 2006;13:1457–1470. doi: 10.1038/sj.gt.3302782. [DOI] [PubMed] [Google Scholar]