Abstract
Microtubules of the mitotic spindle are the basis for chromosome segregation. In metaphase, microtubules show high dynamic instability, thought to aid the ‘search and capture’ of chromosomes for bipolar alignment on the spindle. At anaphase onset microtubules suddenly become more stable, but it is unknown how this change in microtubule behaviour is regulated and what its importance is for the ensuing chromosome segregation1-4. We show here that in the budding yeast Saccharomyces cerevisiae activation of the phosphatase Cdc14 at anaphase onset is required and sufficient to silence microtubule dynamics. Cdc14 is activated by separase, the protease that triggers sister chromatid separation, thus linking anaphase onset to microtubule stabilisation5,6. If sister chromatids separate in the absence of Cdc14 activity, microtubules maintain high dynamic instability, correlating with defects in both the movement of chromosomes to the spindle poles (anaphase A), as well as elongation of the anaphase spindle (anaphase B). Cdc14 promotes localisation of microtubule stabilising proteins to the anaphase spindle, and dephosphorylation of the kinetochore component Ask1 contributes to both the silencing of microtubule turnover and successful anaphase A.
Microtubules are characterised by dynamic instability, repeated cycles of growth and shrinkage. Microtubule turnover in dividing cells increases dramatically as cells progress from interphase into mitosis, promoted by the rise in cyclin-dependent kinase (Cdk) activity7,8. The microtubule cytoskeleton is reorganised to form the bipolar mitotic spindle, and chromosomes attach to the spindle via contacts of the two sister kinetochores with the plus ends of microtubules that emanate from opposite spindle poles. The fast turnover of metaphase spindle microtubules is thought to help correct erroneous attachments that occur during bipolar chromosome alignment. At anaphase onset, when separase cleaves the chromosomal cohesin to separate sister chromatids9, microtubule dynamics suddenly stabilise as the spindle starts to elongate, but Cdk activity only begins to decline.
We analysed microtubule dynamics in metaphase arrested budding yeast cells when chromosome segregation was triggered by ectopic expression of separase9. Microtubules were fluorescently labelled by expression of a GFP-α-tubulin fusion protein10, and the dynamic state of microtubules was assessed by measuring fluorescence recovery after photobleaching (FRAP) of a spindle segment. Bleached segments of metaphase spindles recovered 37% (s. d. 11%, n=6) of their fluorescence within 100 seconds, indicative of rapid microtubule turnover (Fig. 1, and Supplementary Fig. S1). Separase expression triggered chromosome segregation and concomitant stabilisation of microtubules. Fluorescence recovery was significantly reduced to 14% (s. d. 8%, n=8) after bleaching (Fig. 1, and Supplementary Fig. S1), similar to what has been observed during wild type mitosis3. This suggests that separase activation at anaphase onset triggers both chromosome segregation and microtubule stabilisation.
Figure 1.
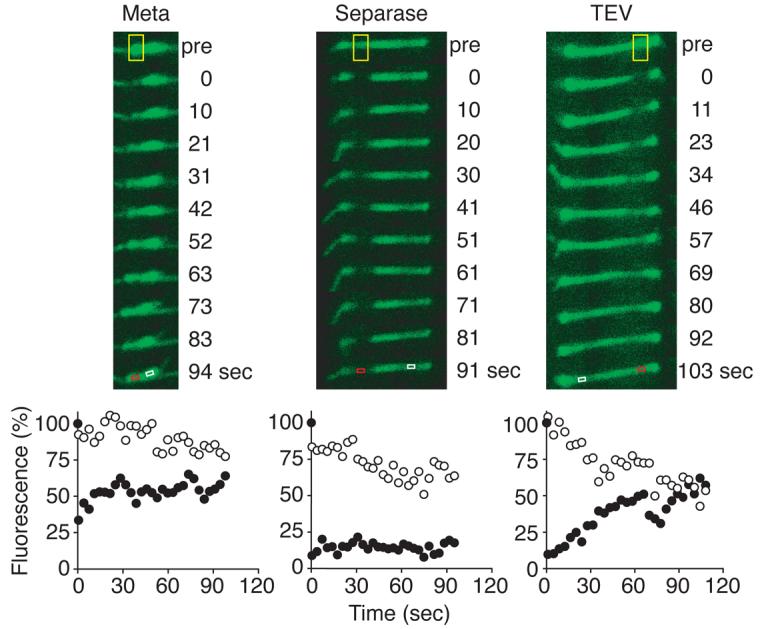
Separase stabilises microtubule dynamics at anaphase onset. A photobleached segment (yellow box) of the GFP-tubulin labelled spindle recovers in strain Y1363 (MATa MET-CDC20 GAL-ESP1 yEGFP-TUB1) arrested in metaphase, indicative of high microtubule turnover, but recovery is reduced after anaphase onset triggered by separase. After TEV protease-triggered anaphase in strain Y1362 (MATα MET-CDC20 GAL-TEV SCC1TEV yEGFP-TUB1) fluorescence recovery remains high. Fluorescence intensity in control (white box, open circles) and bleached (red box, filled circles) regions are plotted relative to their pre-bleach intensity.
Stabilisation of spindle microtubules could be a consequence of sister chromatid separation and spindle elongation. To test this we replaced separase with expression of the foreign TEV protease that is also able to trigger sister chromatid separation by cleavage of accordingly engineered cohesin, while endogenous separase remains bound by its inhibitor securin9. Microtubules of the elongating spindle after TEV protease expression recovered 33% (s. d. 28%, n=9) of their fluorescence, indicating that microtubule dynamics persisted in a metaphase like state (Fig. 1, and Supplementary Fig. S1). Therefore, microtubule stabilisation at anaphase onset is achieved by separase in a reaction that is independent of sister chromatid separation.
Separase, at the same time as cleaving cohesin, activates the phosphatase Cdc14 (Ref. 5,6). We therefore asked whether Cdc14 acts to stabilise microtubule dynamics at anaphase onset. In cells in which Cdc14 had been inactivated due to the temperature sensitive cdc14-1 mutation, anaphase spindle microtubules remained dynamic and bleached segments recovered 35% (s. d. 22%, n=8) of their initial fluorescence (Fig. 2, and Supplementary Fig. S1). This indicates that Cdc14 is required to stabilise spindle microtubules at anaphase onset. We addressed whether Cdc14 activation was sufficient to stabilise microtubule dynamics by ectopic expression of Cdc14 in metaphase arrested cells. After Cdc14 expression, bleached segments recovered 14% (s. d. 16%, n=6) of the initial fluorescence (Fig. 2, and Supplementary Fig. S1), similar to what is normally seen in anaphase. Therefore, Cdc14 activation causes silencing of microtubule dynamics. Ectopic expression of Cdc14 together with TEV protease produced stable elongating spindle microtubules that recovered 6% (s. d. 11%, n=6) fluorescence after photobleaching (Fig. 2, and Supplementary Fig. S1). Morphological spindle abnormalities and premature breakdown have been described during TEV protease-triggered anaphase9. Co-expression with TEV protease of Cdc14 fully restored spindle morphology and prevented breakage (Supplementary Fig. S2). Thus, Cdc14 promotes a change in microtubule dynamics, and together with cohesin cleavage is sufficient for formation of a stable anaphase spindle.
Figure 2.
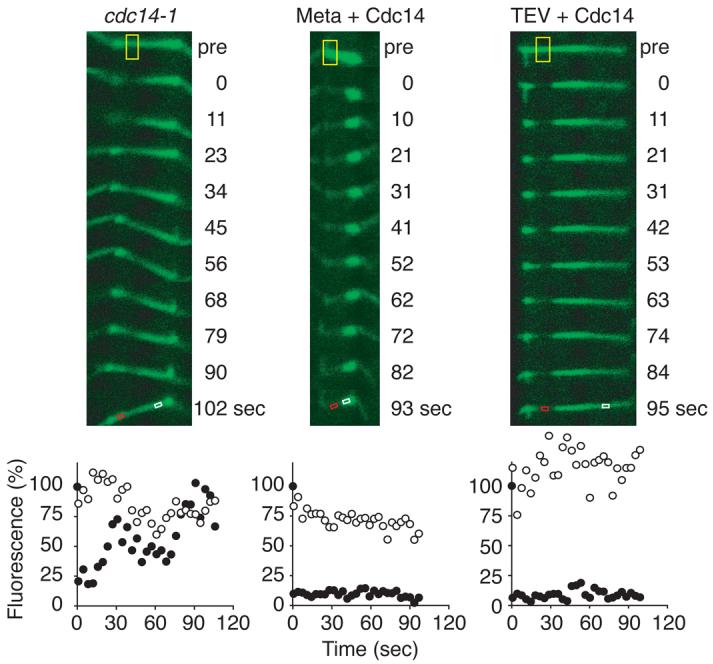
Cdc14 phosphatase regulates microtubule dynamics in mitosis. Anaphase spindles in the absence of Cdc14 activity in strain Y1594 (MATa cdc14-1 TyEGFP-TUB1) maintain high microtubule turnover, and Cdc14 expression in metaphase arrested strain Y1533 (MATα MET-CDC20 GAL-CDC14-Pk yEGFP-TUB1) is sufficient to silence microtubule dynamics. Cdc14 expression together with TEV protease in strain Y1539 (as Y1533, but GAL-TEV SCC1TEV) generates a stable anaphase spindle. See Fig. 1 for key to symbols.
Photobleaching of the elongating anaphase spindle probed the dynamic state of the interpolar microtubules that overlap at the spindle midzone, suggesting that their stability is regulated by Cdc14. We also analysed the dynamic state of microtubule plus ends attached to kinetochores, which can be observed as oscillating movement of GFP-marked sister centromeres in metaphase11. After Cdc14 expression, oscillation of a locus 1.4 kb from centromere V (cenV) was damped and the cenV pair remained separated, close to opposite spindle poles (Supplementary Fig. S3). This suggests that also the turnover of kinetochore microtubules is regulated by Cdc14.
The dependence on Cdc14 activity opened the possibility to study the importance of anaphase microtubule stabilisation for chromosome segregation. We analysed anaphase progression after TEV protease-triggered sister chromatid separation, when Cdc14 is inactive and high microtubule turnover persists. We first analysed the movement of chromosomes towards the spindle poles (anaphase A). cenV was again marked with GFP, in a strain in which also the spindle pole body (SPB) was labelled by a Spc42-GFP fusion protein12. In metaphase, the two cenV-GFP signals were seen between the two SPBs in most cells (Fig. 3a). When, as a control, separase was expressed, the two cenV signals moved close to or merged with opposite SPBs in binucleate anaphase cells. In contrast, cenV signals often remained at a greater distance from SPBs in binucleate cells after expression of TEV protease. We also analysed kinetochore distribution in anaphase by staining the kinetochore component Mtw1 (Ref. 11). In all separase expressing control anaphase cells, one compact cluster of Mtw1 was found adjacent to each SPB (Fig. 3b). Only 34% of SPBs after TEV protease triggered anaphase showed one Mtw1 signal with the majority (66%) showing multiple, scattered Mtw1 foci. Co-expression with TEV protease of Cdc14 rescued the movement of Mtw1 into one focus adjacent to the SPBs in 80% of cells (Fig 3b). This indicates that Cdc14 activation is required for successful anaphase A, and suggests that persistent high microtubule turnover at anaphase onset interferes with transport of chromosomes towards the spindle poles.
Figure 3.
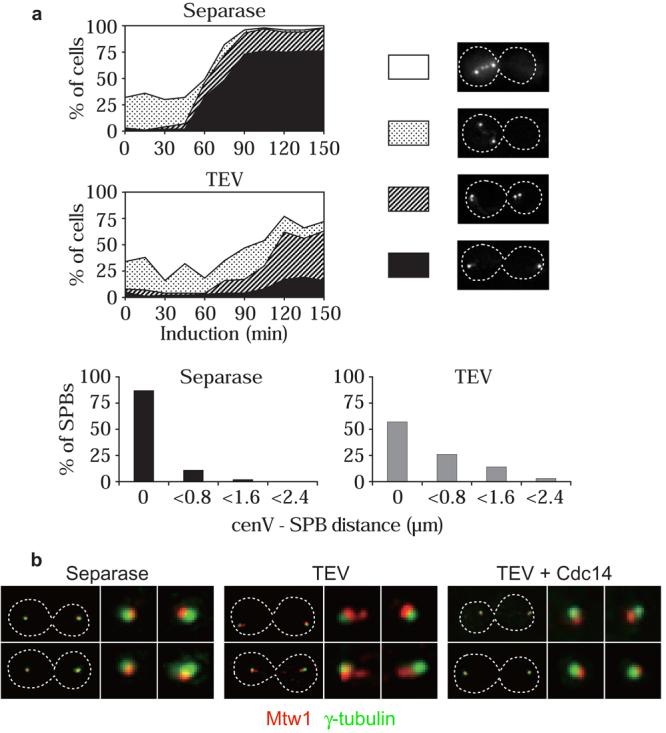
Cdc14 activation is required for successful anaphase A. a, Movement of cenV to the SPBs (anaphase A) during separase-triggered anaphase in strain Y1887 (MATa MET-CDC20 GAL-ESP1 cenV-tetOs TetR-GFP SPC42-GFP), and after TEV protease-triggered chromosome segregation in strain Y1888 (MATα MET-CDC20 GAL-TEV SCC1TEV cenV-tetOs TetR-GFP SPC42-GFP). Cells were stained with DAPI. The percentage of uninucleate cells with cenV between the SPBs (white area) or indistinguishable from the SPBs (dotted), and of binucleate cells with at least one cenV signal distant from (hatched), or both overlapping with the SPBs (black), is presented. CenV distances from the nearest SPB after 120 min are shown in detail. b, Movement of kinetochores to SPBs in anaphase is promoted by Cdc14. Mtw1-HA and γ-tubulin distribution was analysed in fixed binucleate cells (strains Y1900 (MATα MET-CDC20 GAL-ESP1 MTW1-HA6), Y1901 (MATα MET-CDC20 GAL-TEV SCC1TEV MTW1-HA6), and Y1902 (as Y1901 but also GAL-CDC14).
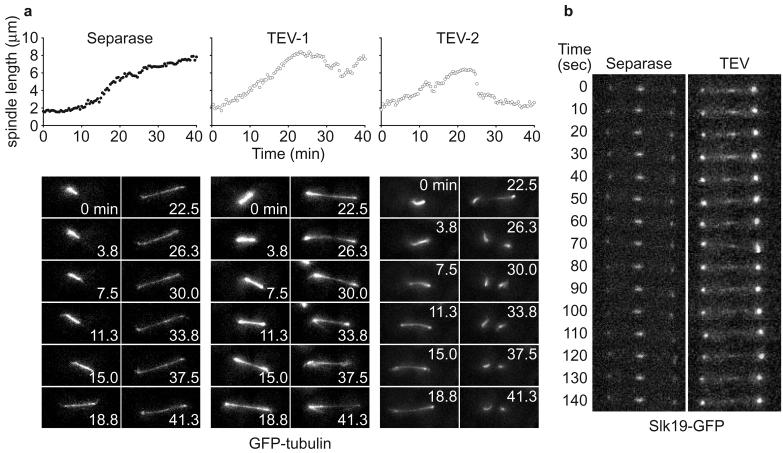
We then analysed elongation of the anaphase spindle (anaphase B). After separase expression in metaphase arrested cells, spindle elongation proceeded with biphasic kinetics, similar to what has been described during wild type anaphase (Fig. 4a, Supplementary Movie 1, and Ref. 10). Anaphase B after TEV protease-triggered sister chromatid separation differed in several aspects. The rate of elongation was variable, reaching up to 0.45 μm/min for short periods (similar to the initial fast elongation phase in wild type), but proceeding slower at most times. Elongation was discontinuous and, in 5 out of 6 cells observed, spindle length receded before further elongation resumed (Fig. 4a TEV-1, and Supplementary Movie 2). In 2 cases the spindle broke down before reaching full anaphase length (Fig. 4a TEV-2, and Supplementary Movie 3), a situation incompatible with complete chromosome segregation.
Figure 4.
Anaphase B defects during TEV protease-triggered anaphase. a, pole to pole distance during spindle elongation was measured in 3D, and projections are shown, of the GFP-tubulin signal during separase-triggered (Y1363) and two examples of TEV protease-triggered anaphase (Y1362). b, the spindle midzone, visualised by Slk19-GFP, is stable during separase-induced anaphase in strain Y357 (as Y1363, but SLK19-GFP), but fluctuates during TEV protease-induced anaphase in strain Y406 (as Y1362, but SLK19-GFP).
The elongating budding yeast anaphase spindle consists of 2-3 microtubules from each spindle pole, interdigitating at the spindle midzone13. Persisting dynamic instability of these microtubules could lead to the observed fluctuations in spindle elongation and stability. Additional evidence that the interdigitating microtubules maintained dynamic instability came from the observation of Slk19, a protein marking microtubule plus ends at both kinetochores and the spindle midzone14. Time lapse imaging of Slk19 showed a stable midzone signal after separase-triggered anaphase. In contrast, Slk19 was distributed over a broad zone of the TEV protease-induced anaphase spindle and the signal fluctuated laterally over time (Fig 4b). If persistent dynamic instability of interpolar microtubules was responsible for the observed anaphase B defects, deletion of the budding yeast kinesin Kip3, implicated in microtubule destabilisation15, might partly rescue the defect. Spindle breakage after TEV protease-induced anaphase was reduced in the absence of Kip3, even though anaphase spindles still appeared morphologically abnormal, often lacking a discernable midzone structure (Supplementary Fig. S4). Persistent dynamic instability might thus be responsible for spindle breakage. Cdc14 could in addition regulate proteins involved in midzone formation16. Cdc14 activity is also required to resolve sister chromatids at the rDNA locus on chromosome XII, but persisting rDNA cohesion does not itself cause anaphase defects when rDNA segregation is prevented by inactivation of topoisomerase II or condensin17.
How does Cdc14 regulate microtubule dynamics at anaphase onset? The Sli15/INCENP subunit of the aurora B kinase complex is dephosphorylated by Cdc14 in early anaphase16,18 to target the complex to the spindle. A phospho-site mutant that allows Cdc14-independent spindle localisation16 reduced microtubule dynamics in metaphase (Supplementary Fig. S1), and partly but not fully restored anaphase B progression after TEV protease expression (Ref. 16, and our unpublished results). Ask1 is another mitotic phospho-protein, dephosphorylated by Cdc14 in early anaphase18,19. Ask1 is part of the DASH kinetochore complex that is thought to regulate microtubule turnover at kinetochores. Mutation of two Cdk phosphorylation sites (Ask1-2A) largely reduces its mitotic phosphorylation19, and cells expressing Ask1-2A as their only source of Ask1 showed greatly reduced microtubule dynamics in metaphase (Fig. 5a, and Supplementary Fig. S1). Ask1-2A also improved kinetochore anaphase A movement during TEV protease triggered anaphase. In early anaphase (spindle length 5-7 μm), a single Mtw1 focus was seen close to only 17% of SPBs in TEV protease expressing cells, a fraction that was doubled to 34% of SPBs in Ask1-2A cells (Fig. 5a). This suggests that Ask1 dephosphorylation at anaphase onset contributes to silencing of microtubule dynamics and successful anaphase A.
Figure 5.
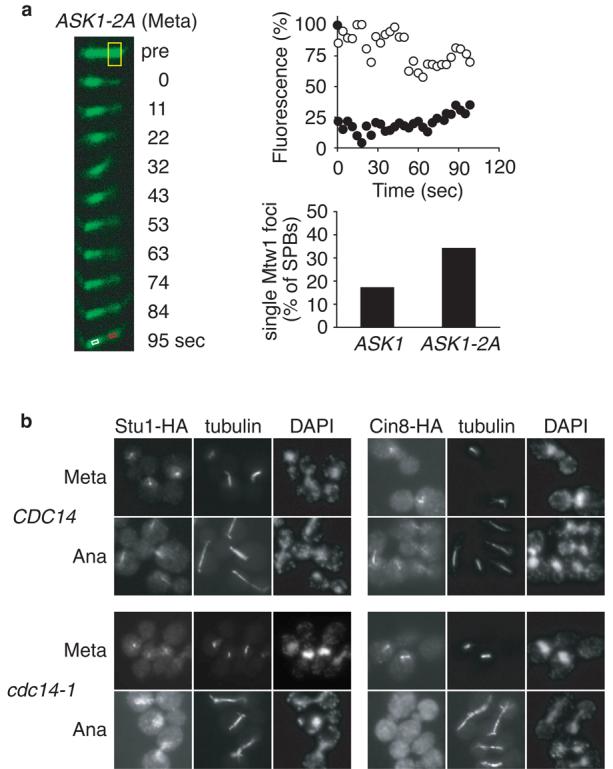
Proteins involved in the Cdc14 response. a, Ask1-2A, lacking two Cdc28 phosphorylation sites, confers reduced microtubule dynamics in metaphase, and improves anaphase A in the absence of Cdc14. FRAP was measured in metaphase arrested strain Y1893 (as Y1362, but ASK1-S216,250A). Mtw1 congression into a single focus close to SPBs was analysed in early anaphase (spindle length 5-7 μm) in strains Y1901 and Y2104 (as Y1901, but ASK1-S216,250A). b, anaphase spindle localisation of Stu1 and Cin8 depends on Cdc14. Immunofluorescence in metaphase arrested cells 30 min after shift to 37°C, a non-permissive temperature for cdc14-1, and after release into anaphase at 37°C. Strains Y1587 (MATa GAL-CDC20 STU1-HA6), Y1588 (as Y1587, but cdc14-1), Y1618 (MATa GAL-CDC20 CIN8-HA6), and Y1619 (as Y1618, but cdc14-1).
Additional Cdc14 targets that regulate microtubule dynamics may include kinesins and other microtubule associated proteins. The budding yeast Kip3 kinesin, and the TOG/XMAP215 homolog Stu2 promote microtubule turnover 15,20,21. Both Kip3 and Stu2 are mitotic phosphoproteins but do not appear to be regulated by Cdc1418. The essential microtubule associated protein Stu1 promotes spindle stability in budding yeast22. It is the homolog of human CLASP1 that associates with growing microtubule plus ends and is involved in the regulation of microtubule dynamics23. We found that Stu1 association with anaphase microtubules depends on Cdc14 (Fig. 5b). Thus, Cdc14-dependent Stu1 localisation to the anaphase spindle may contribute to the downregulation of dynamic instability at anaphase onset. C. elegans Cdc14 dephosphorylates the bidirectional kinesin ZEN-4 to support microtubule bundling at the spindle midzone24. We found that spindle localisation of the BimC family kinesin Cin8 also depends on Cdc14 (Fig. 5b). BimC kinesin promotes antiparallel microtubule sliding during spindle elongation25, and its absence could contribute to the observed anaphase B defects.
We present here the genetic dissection of the downregulation of microtubule dynamics at anaphase onset. We find that not sister chromatid separation but activation of the phosphatase Cdc14 by separase is responsible for the reduction in microtubule turnover. This allowed us to study the importance of this regulation at anaphase onset. When anaphase is triggered without Cdc14 activation, chromosome segregation begins, but the completion of anaphase A as well as anaphase B is hampered. Cdc14 counteracts Cdk activity, and without Cdc14 anaphase proceeds in the presence of higher than normal Cdk activity. This by itself is unlikely to prevent microtubule stabilisation, as no anaphase spindle defects have been reported due to stable or increased levels of B-type cyclins26,27. Strong overexpression of cyclin B may interfere with Cdc14 activity and indeed has been shown to cause anaphase defects that could be explained by misregulation of microtubule dynamics28,29. A role for a phosphatase in the elongation of anaphase spindles in Xenopus egg extracts has been postulated30, although the identity of the phosphatase remained unclear. We now suggest that at least in budding yeast activation of Cdc14 phosphatase at anaphase onset gives the impetus to stabilise microtubule dynamics. The interplay between Cdk activity and counteracting phosphatases during anaphase will be interesting to analyse in further detail.
Methods
Yeast strains and plasmids
All strains were derivatives of W303. Epitope tagging of endogenous genes was performed by gene targeting using polymerase chain reaction products. GAL1 promoter driven expression of separase, TEV protease, or Cdc14 in cells arrested in metaphase by Cdc20 depletion under control of the MET3 promoter was as described9,17. The original plasmid for expression of the GFP-Tub1 fusion protein was a kind gift from A. Straight10. The GFP-coding sequence in this plasmid was changed to include S65G and V72A mutations (yEGFP), or additional V163A and S175G mutations (TyEGFP) to enhance its fluorescence intensity and thermo resistance, respectively. For live cell microscopy, cultures were grown in YNB medium containing 3% raffinose and 120 μg/ml of auxotrophic supplements except methionine. Metaphase arrest was achieved by addition of 200 mM methionine, and the GAL1 promoter was induced by adding 2% galactose. To analyse microtubule dynamics in cdc14-1 cells, cultures grown in YNB medium containing 2% glucose were synchronised in G1 phase by pheromone α-factor treatment, and released at 37°C. Strains containing GFP dots close to cenV and Spc42 fused to GFP were as described12. Strains carrying the ASK1-2A allele19 were a kind gift from S. Elledge.
Microscopy
Cells were mounted on glass bottom culture dishes (MatTek), coated with concanavalin A. FRAP experiments were conducted on an inverted Zeiss LSM510 confocal microscope, using a 63x/1.40 NA objective lens and a 200 nm pin-hole, the pixel width was 0.035 μm. After photobleaching, images were taken every 3 seconds as average of 4 scans. Only images in which the spindle remained in focus throughout the observation period were further analysed. Fluorescence intensity in bleached and control regions was measured in areas covering approximately 50 pixels using ImageJ software (NIH). Time-lapse observations of GFP-Tub1 and Slk19-GFP were performed on a DeltaVision Olympus IX70 inverted microscope using a 60x/1.40 NA objective lens. To analyse spindle elongation, 14 z-sections (0.2 μm interval) were taken every 25 seconds, Slk19 was filmed in 10 second intervals (8 z-sections, 0.2 μm interval). Images were analysed using SoftWoRx (Applied Precision). Indirect immunofluorescence was performed following standard procedures, Mtw1 localisation close to SPBs was analysed in 3D deconvolved images acquired with a 100x/1.40 NA objective. Antibodies were anti-HA (16B12, BAbCO) and anti-α-tubulin (YOL1/34, Serotec). The anti-γ-tubulin serum was a kind gift from J. Kilmartin.
Supplementary Material
Supplementary Information accompanies the paper on Nature's website (http://www.nature.com).
Acknowledgements
We thank S. Elledge, J. Kilmartin, and A. Straight for reagents, R. Carazo-Salas and A. Nicol for advise on microscopy, J. Cau. T. Davis, A. Hyman, T. Toda and all members of our laboratory for helpful discussions and critical reading of the manuscript, and in particular M. Sullivan for his help at the outset of this study.
References
- 1.Zhai Y, Kronebusch PJ, Borisy GG. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J. Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallavarapu A, Sawin K, Mitchison T. A switch in microtubule dynamics at the onset of anaphase B in the mitotic spindle of Schizosaccaromyces pombe. Curr. Biol. 1999;9:1423–1426. doi: 10.1016/s0960-9822(00)80090-1. [DOI] [PubMed] [Google Scholar]
- 3.Maddox PS, Bloom KS, Salmon ED. The polarity and dynamics of microtubule assembly in the budding yeast Saccharomyces cerevisiae. Nat. Cell Biol. 2000;2:36–41. doi: 10.1038/71357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kline-Smith SL, Walczak CE. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol. Cell. 2004;15:317–327. doi: 10.1016/j.molcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Stegmeier F, Visintin R, Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. doi: 10.1016/s0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan M, Uhlmann F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat. Cell Biol. 2003;5:249–254. doi: 10.1038/ncb940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- 8.Verde F, Labbé J-C, Dorée M, Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature. 1990;343:233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- 9.Uhlmann F, Wernic D, Poupart M-A, Koonin EV, Nasmyth K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell. 2000;103:375–386. doi: 10.1016/s0092-8674(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 10.Straight AF, Marshall WF, Sedat JW, Murray AW. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science. 1997;277:574–578. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- 11.Goshima G, Yanagida M. Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell. 2000;100:619–633. doi: 10.1016/s0092-8674(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Fuchs J, Loidl J, Nasmyth K. Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nat. Cell Biol. 2000;2:492–499. doi: 10.1038/35019529. [DOI] [PubMed] [Google Scholar]
- 13.Winey M, et al. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 1995;129:1601–1615. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng X, et al. Slk19p is a centromere protein that functions to stabilize mitotic spindles. J. Cell Biol. 1999;146:415–425. doi: 10.1083/jcb.146.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RK, et al. The kinesin-related proteins, Kip2p and Kip3p, function differently in nuclear migration in yeast. Mol. Biol. Cell. 1998;9:2051–2068. doi: 10.1091/mbc.9.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira G, Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302:2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan M, Higuchi T, Katis VL, Uhlmann F. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell. 2004;117:471–482. doi: 10.1016/s0092-8674(04)00415-5. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan M, Hornig NCD, Porstmann T, Uhlmann F. Studies on substrate recognition by the budding yeast separase. J. Biol. Chem. 2004;279:1191–1196. doi: 10.1074/jbc.M309761200. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Elledge SJ. The DASH complex component Ask1 is a cell cycle-regulated Cdk substrate in Saccharomyces cerevisiae. Cell Cycle. 2003;2:143–148. [PubMed] [Google Scholar]
- 20.Kosco KA, et al. Control of microtubule dynamics by Stu2p is essential for spindle orientation and metaphase chromosome alignment in yeast. Mol. Biol. Cell. 2001;12:2870–2880. doi: 10.1091/mbc.12.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Breugel M, Drechsel D, Hyman A. Stu2p, the budding yeast member of the conserved Dis1/XMAP215 family of microtubule-associated proteins is a plus end-binding microtubule destabilizer. J. Cell Biol. 2003;161:359–369. doi: 10.1083/jcb.200211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin H, You L, Pasqualone D, Kopski KM, Huffaker TC. Stu1p is physically associated with β-tubulin and is required for structural integrity of the mitotic spindle. Mol. Biol. Cell. 2002;13:1881–1892. doi: 10.1091/mbc.01-09-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiato H, et al. Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell. 2003;113:891–904. doi: 10.1016/s0092-8674(03)00465-3. [DOI] [PubMed] [Google Scholar]
- 24.Mishima M, Pavicic V, Grüneberg U, Nigg EA, Glotzer M. Cell cycle regulation of central spindle assembly. Nature. 2004;430:908–913. doi: 10.1038/nature02767. [DOI] [PubMed] [Google Scholar]
- 25.Straight AF, Sedat JW, Murray AW. Time-lapse microscopy reveals unique roles for kinesins during anaphase in budding yeast. J. Cell Biol. 1998;143:687–694. doi: 10.1083/jcb.143.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surana U, et al. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray AW, Desai AB, Salmon ED. Real time observation of anaphase in vitro. Proc Natl Acad Sci USA. 1996;93:12327–12332. doi: 10.1073/pnas.93.22.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheatley SP, et al. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J. Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parry DH, Hickson GRX, O'Farrell PH. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr. Biol. 2003;13:647–653. doi: 10.1016/s0960-9822(03)00242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tournebize R, et al. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 1997;16:5537–5549. doi: 10.1093/emboj/16.18.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.