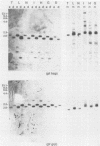
Abstract
Heat shock-related stress proteins present in all eucaryotes and procaryotes have been shown to be immune targets in a broad range of infections. We have analyzed sera from people exposed primarily to Plasmodium falciparum for specific antibodies against two heat shock-related proteins (proteins similar to the heat shock protein with a molecular weight of 75,000 [Pfhsp] and a glucose-regulated protein with a molecular weight of 72,000 [Pfgrp]). In an immunoprecipitation analysis with metabolically labeled parasites and synthetic peptides in an enzyme-linked immunosorbent assay, specific antibodies against Pfhsp and Pfgrp were detected in the sera of these individuals. Sera from people exposed to a different human malarial parasite, Plasmodium vivax, did not react with the peptides in an enzyme-linked immunosorbent assay. Southern blot analysis with DNA isolated from P. falciparum from different geographical locations showed a conservation of genes for these stress proteins; thus, they are likely to be immune targets in various endemic areas. Lymphocytes from two tested immune donors responded in proliferation assays to purified Pfhsp and Pfgrp and purified recombinant proteins. However, a similar response was also seen in lymphocytes from nonimmune individuals and has raised questions pertaining to a generalized responsiveness of lymphocytes to some common determinants present in heat shock-related proteins in various pathogens.
Full text
PDF

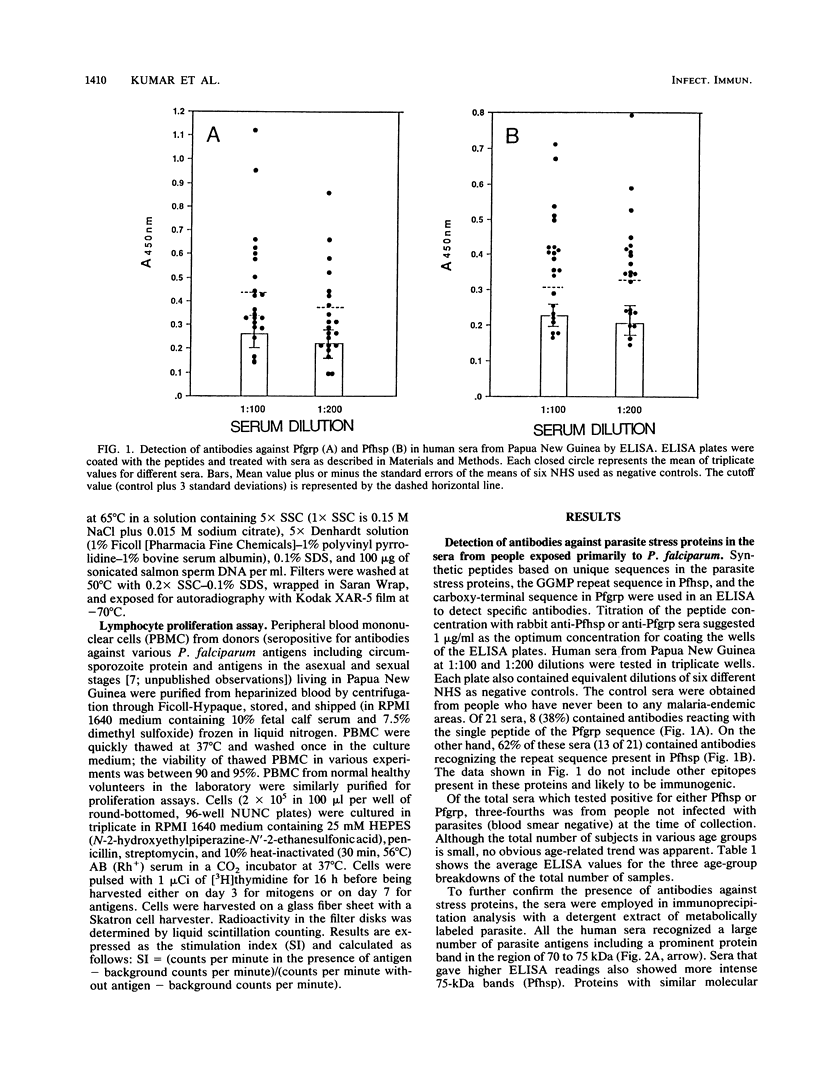




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Ardeshir F., Flint J. E., Richman S. J., Reese R. T. A 75 kd merozoite surface protein of Plasmodium falciparum which is related to the 70 kd heat-shock proteins. EMBO J. 1987 Feb;6(2):493–499. doi: 10.1002/j.1460-2075.1987.tb04780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A. E., Favaloro J. M., Burkot T. R., Culvenor J. G., Crewther P. E., Brown G. V., Anders R. F., Coppel R. L., Kemp D. J. A repetitive antigen of Plasmodium falciparum that is homologous to heat shock protein 70 of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8713–8717. doi: 10.1073/pnas.83.22.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Good M. F., Kumar S., Miller L. H. The real difficulties for malaria sporozoite vaccine development: nonresponsiveness and antigenic variation. Immunol Today. 1988 Nov;9(11):351–355. doi: 10.1016/0167-5699(88)91336-9. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Carter R., Burkot T. R., Quakyi I. A., Kumar N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 1988 Mar;10(2):209–218. doi: 10.1111/j.1365-3024.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Haire R. N., Peterson M. S., O'Leary J. J. Mitogen activation induces the enhanced synthesis of two heat-shock proteins in human lymphocytes. J Cell Biol. 1988 Mar;106(3):883–891. doi: 10.1083/jcb.106.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kumar N., Syin C. A., Carter R., Quakyi I., Miller L. H. Plasmodium falciparum gene encoding a protein similar to the 78-kDa rat glucose-regulated stress protein. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6277–6281. doi: 10.1073/pnas.85.17.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Hansen J. L., Dame J. B., Mullins J. A. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. doi: 10.1126/science.6330899. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Newport G., Culpepper J., Agabian N. Parasite heat-shock proteins. Parasitol Today. 1988 Nov;4(11):306–312. doi: 10.1016/0169-4758(88)90111-1. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Shinnick T. M., Houghten R. A., Kvalheim G., Degre M., Lundin K. E., Godal T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J Immunol. 1988 Oct 15;141(8):2749–2754. [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Crewther P. E., Thompson J. K., Corcoran L. M., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. A second antigenic heat shock protein of Plasmodium falciparum. DNA. 1988 Mar;7(2):71–78. doi: 10.1089/dna.1988.7.71. [DOI] [PubMed] [Google Scholar]
- Polla B. S. A role for heat shock proteins in inflammation? Immunol Today. 1988 May;9(5):134–137. doi: 10.1016/0167-5699(88)91199-1. [DOI] [PubMed] [Google Scholar]
- Quakyi I. A., Carter R., Rener J., Kumar N., Good M. F., Miller L. H. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987 Dec 15;139(12):4213–4217. [PubMed] [Google Scholar]
- Sargent C. A., Dunham I., Trowsdale J., Campbell R. D. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1968–1972. doi: 10.1073/pnas.86.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Abdulla E. Down-regulation of IL-1 beta biosynthesis by inducers of the heat-shock response. J Immunol. 1988 Sep 15;141(6):2027–2034. [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Yang Y. F., Tan-ariya P., Sharma Y. D., Kilejian A. The primary structure of a Plasmodium falciparum polypeptide related to heat shock proteins. Mol Biochem Parasitol. 1987 Nov;26(1-2):61–67. doi: 10.1016/0166-6851(87)90130-7. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]