Abstract
The Gaussian curvature elastic energy contribution to the energy of membrane fusion intermediates has usually been neglected because the Gaussian curvature elastic modulus, κ, was unknown. It is now possible to measure κ for phospholipids that form bicontinuous inverted cubic (QII) phases. Here, it is shown that one can estimate κ for lipids that do not form QII phases by studying the phase behavior of lipid mixtures. The method is used to estimate κ for several lipid compositions in excess water. The values of κ are used to compute the curvature elastic energies of stalks and catenoidal fusion pores according to recent models. The Gaussian curvature elastic contribution is positive and similar in magnitude to the bending energy contribution: it increases the total curvature energy of all the fusion intermediates by 100 units of kBT or more. It is important to note that this contribution makes the predicted intermediate energies compatible with observed lipid phase behavior in excess water. An order-of-magnitude fusion rate equation is used to estimate whether the predicted stalk energies are consistent with the observed rates of stalk-mediated processes in pure lipid systems. The current theory predicts a stalk energy that is slightly too large, by ∼30 kBT, to rationalize the observed rates of stalk-mediated processes in phosphatidylethanolamine or N-monomethylated dioleoylphosphatidylethanolamine systems. Despite this discrepancy, the results show that models of fusion intermediate energy are accurate enough to make semiquantitative predictions about how proteins mediate biomembrane fusion. The same rate model shows that for proteins to drive biomembrane fusion at observed rates, they have to perform mediating functions corresponding to a reduction in the energy of a purely lipidic stalk by several tens of kBT. By binding particular peptide sequences to the monolayer surface, proteins could lower fusion intermediate energies by altering the elastic constants of the patches of lipid monolayer that form the stalk. Here, it is shown that if peptide binding changes κ or some other combinations of local elastic constants by only tens of percents, the stalk energy and the energy of catenoidal fusion pores would decrease by tens of kBT relative to the pure lipid value. This is comparable to the required mediating effect. The curvature energies of stalks and catenoidal fusion pores have almost the same dependence on monolayer elastic constants as the curvature energies of the rhombohedral and QII phases; respectively. The effects of isolated fusion-relevant peptides on the energies of these intermediates can be determined by studying the effects of the peptides on the stability of rhombohedral and QII phases.
INTRODUCTION
The structures of intermediates in the process of biomembrane fusion are difficult to establish, since the intermediates are small and transient. However, using information obtained from the study of model lipid systems, it is possible to estimate the energy of different proposed intermediate structures relative to the initial flat bilayer state. This energy is taken to represent the minimum activation energy for membrane fusion. It is thus possible to postulate mechanisms for membrane fusion by looking for mechanisms in which the intermediate structures have low free energy and change with lipid composition in a fashion compatible with the observed composition-dependent membrane fusion rates. To date, no fusion mechanism has been proposed that proceeds via lower-energy structures than the recent versions of the stalk mechanism (1–4). In particular, the mechanism described by Kozlovsky and colleagues (2,3) has been successful in rationalizing many qualitative observations concerning the lipid composition dependence of membrane fusion rates (5–7), and seems compatible with many observations of protein-mediated biomembrane fusion (6–9).
The principal components of the energies of a fusion intermediate like a stalk are the energy necessary to closely oppose the surfaces of the two original bilayers (10,11); the curvature elastic energies of the lipid monolayers; and the energy associated with stabilization of hydrophobic defects (1,12). The latter contribution arises through local variations in lipid acyl chain length or in lipid molecule tilt (2). The curvature energies of intermediates in fusion can be calculated using the Helfrich expression for the lipid monolayer curvature energy (13). The Helfrich curvature energy is composed of contributions from the bending energy and the Gaussian curvature elastic energy.
Most authors have either neglected the contribution of the Gaussian curvature elastic energy in calculating the curvature energy of fusion intermediates (1–4,12), or have assumed that the contribution is very small (14). An awkward feature of some of these theories is that their predictions contradict observed lipid phase behavior. Some predict the existence of lipid phases not previously observed for some ranges of lipid spontaneous curvature, such as phases composed of stalks for sufficiently negative values of the spontaneous curvature (1,2), or inverted cubic phases for any lipid with negative spontaneous curvature above the chain-melting temperature (14), where only lamellar phases are observed. The theory of May (4) deals only with stalks. It predicts stalk energies that are >0 for values of spontaneous curvature >∼−0.35 nm−1, and hence is compatible with the observed absence of stable stalk phases in pure PE and lipids with more positive curvatures in excess water.
One of the reasons for calculating the energy of fusion intermediates is to help us understand how proteins mediate membrane fusion in vivo. For our models to be useful for this purpose, the predictions must be compatible with two sets of observations on pure lipid systems. First, they must be compatible with observed lipid phase behavior. If they do not pass this test, then they are incorrectly predicting the curvature energy of monolayer assemblies. Second, the predicted fusion intermediate energies must be compatible with the observed rates of lipid mixing and fusion in pure lipid systems. It is therefore important to calculate the energies of fusion intermediates containing the Gaussian curvature energy contribution, and determine whether the results are consistent with these observations.
A recent theoretical study (11) showed that inclusion of the Gaussian curvature contribution is necessary to rationalize the lipid composition and water activity ranges of stability of the rhombohedral phase, in which the structural unit is essentially a stalk fusion intermediate. In Kozlovsky et al. (11), the contribution of the Gaussian curvature elastic energy to the stalk energy is positive and comparable to that of the bending in absolute magnitude. These results imply that the Gaussian curvature elastic energy is a substantial component of the curvature energy of fusion intermediates, and that neglecting it produces substantial under-estimates of intermediate energies relative to planar bilayers.
To calculate the Gaussian curvature contribution to the curvature energy, the Gaussian curvature elastic modulus must be known (13), and this modulus is lipid composition-dependent (15,16). Recently, accurate methods for measuring the Gaussian curvature modulus have been developed (17–19), although they have so far been applied to only two lipid compositions. One factor limiting wider application of these methods is that they can only be used on lipid compositions that adopt bicontinuous inverted cubic (QII) phases. Here, the method employed by Siegel (19) is extended to estimate the Gaussian curvature elastic moduli of additional lipid compositions that are more representative of biomembrane lipids. Observations of lipid phase behavior from earlier x-ray diffraction and 31P NMR studies, along with data previously obtained with N-monomethylated dioleoylphosphatidylethanolamine (DOPE-Me (20)), are used to estimate the moduli for DOPE, dioleoylphosphatidylcholine (DOPC), and an equimolar mixture of DOPC and cholesterol.
The derived values of the Gaussian curvature moduli results are then used to calculate the curvature energy for stalks and catenoidal fusion pores for several lipid compositions using recently derived models (11,18). It is shown that the Gaussian curvature elastic energy contribution is positive and of the same order of magnitude as the bending energy contribution for stalks, hemifusion diaphragms, and catenoidal fusion pores in all of the lipid compositions. The Gaussian curvature elastic contribution makes the total curvature energy of the fusion intermediates larger than values obtained using only the bending energy by 100–200 units of kBT, where kB is the Boltzmann constant and T is the temperature. The resulting intermediate energies are compatible with the observed phase behavior of these lipids in excess water. An order-of-magnitude estimate of stalk formation rates in pure lipid systems is used to determine whether the curvature energies of stalks, including the Gaussian curvature elastic energy contributions, are consistent with the observed rates of stalk-mediated processes in pure lipid systems. We presume that membrane fusion-mediating proteins act in part by reducing the energy necessary to create fusion intermediates. To estimate the extent of this reduction, we use the same model of stalk formation rates that would be required for stalks to form on the observed timescale of membrane fusion in two representative biomembrane systems.
THEORY
Fig. 1 is a schematic diagram of the stalk-mediated fusion of two unilamellar bilayer liposomes (5,6). The monolayers of the bilayers are depicted as continuous slabs. The two original liposomes interact through formation of a stalk between the proximal monolayers (Fig. 1 A), which, at the narrowest point in a plane parallel to the original bilayers, has a radius equal to that of one lipid monolayer thickness. The stalk expands in the plane of the bilayers into a hemifusion diaphragm (HF) (Fig. 1 B), which corresponds to a stalk with a disk of planar bilayer inserted in the center. According to a recent model (3), radial expansion of the stalk is spontaneous for lipids with negative values of spontaneous curvature (Js), like DOPE, but requires an applied membrane tension for lipids with larger (less negative) values, like DOPC. The planar bilayer in the center of the HF can form a fusion pore (Fig. 1 C) within the single bilayer diaphragm. It is not clear what the shape of the fusion pore is or where it will form in the HF, although it likely forms at the edge of the diaphragm (3). The pore in the HF is unstable due to the high curvature energy of the pore edges (∼3 kBT/nm of edge length (6)). We presume that the system rearranges by a combination of radial contraction of the rim of the HF and radial expansion of the fusion pore in the planar bilayer expansion to form a more stable bilayer-walled pore (Fig. 1 D). Here, the bilayer-walled pore is referred to as the catenoidal fusion pore. This is because when the two monolayers have the same composition, the minimal-curvature energy form of this pore is achieved when the bilayer midplanes lie on a catenoid (18), which is a zero-curvature surface. The catenoidal fusion pore has also been referred to as an interlamellar attachment (18,19), although earlier uses of this term referred to bilayer-walled pores with a noncatenoidal shape(e.g., (12,14)). It has been proposed that the first fusion pore can also form directly from the stalk, as well as from the HF, based on the results of fluorescence assays in polyethylene glycol (PEG)-induced fusion (21) and coarse-grain computer simulations (22).
FIGURE 1.
Schematic representation of fusion according to the stalk theory (2,3). The monolayers of the bilayers are depicted as slabs. All structures are axially symmetric and appear in the cross section that contains their vertical axes (dotted lines). The first structure to form that bridges two opposed membranes is the stalk (A). For sufficiently negative values of Js (3), or in the presence of sufficient membrane tension, the stalk can expand radially to form a hemifusion diaphragm (B), which contains a disk of planar bilayer membrane in the center. Fusion occurs when a pore forms within this single bilayer (C). The axis of the pore is depicted as the shorter vertical dashed line. The edge of the bilayer pore is unstable, and the system can lower its free energy by forming a catenoidal fusion pore (D).
The HF-to-catenoidal pore rearrangement process must require a substantial input in energy in the form of applied tension in systems with low Js, since radial contraction of the HF rim is not spontaneous (3). In fusion involving planar lipid bilayers made with alkane solvents (23), it is possible that residual solvent lowers the defect stabilization energy inherent to the HF rims (12), but this does not occur in phospholipid unilamellar vesicles made via extrusion (24). Catenoidal fusion pores form in 0.1- to 0.2-μm extruded unilamellar vesicles of DOPE-Me within milliseconds after they are temperature-jumped above the bilayer/nonbilayer phase transition temperature (24). Thus, it is possible that fusion pore formation can occur after limited expansion of the HF (Fig. 1 C) in protein-free, solvent-free membranes (12). In this work, due to the uncertainty in the structure and dimensions of the initial fusion pore, only the curvature energy of the catenoidal fusion pore (Fig. 1 D) will be calculated, because the geometry is more constrained.
The curvature elastic energy per unit area of a continuous monolayer with respect to a planar monolayer is given as (13)
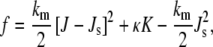 |
(1) |
where J is the monolayer curvature at the monolayer neutral plane, Js the spontaneous curvature at the same plane, km the monolayer bending modulus, and κ the monolayer Gaussian modulus. The curvature is represented by J = c1 + c2 and the Gaussian curvature by K = c1c2, where c1 and c2 are the two principal radii of curvature of the monolayer neutral plane. The sign of κ for lipid monolayers is negative to be consistent with observed lipid phase behavior. In bicontinuous QII phases, c1 and c2 are of different sign, and K < 0. If κ is ≥0, it can be shown that QII phases form instead of lamellar phases for all lipids with even slightly negative values of Js (18), like DOPC, and the QII phases immediately collapse to very small values of the unit cell constant to maximize the area density of K.
Models for stalk structure composed of smooth monolayers predict high curvature energies for stalks (12,14) because of the attendant creation of hydrophobic interstices at the juncture where the hydrophobic surfaces of the monolayers separate. These interstices must be stabilized by entropically disfavored stretching of the surrounding acyl chains, which substantially raises the free energy of the stalk (12). Models that allow for variation in lipid molecule tilt within the monolayers predict lower energies (1–4,25), because this permits the monolayers to form nonsmooth interfaces, which make these interstices unnecessary. Crudely, the monolayers can form “joints” at which the monolayer curvature makes a discontinuous change, as in the center of the stalk structure in Fig. 1 A. The curvature and tilt energy per unit area is then given by (11)
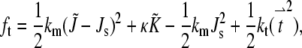 |
(2) |
where t characterizes the tilt of a molecule away from the local surface normal vector, and kt is a tilt elastic constant. kt cannot be measured directly. An estimate (26) of 0.001 kBT/nm2 was used in previous studies (2–4,11), although a more recent estimate (27) places the value at twice the original value. 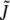 and
and 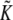 are the splay and saddle splay, respectively, which include additive contributions from monolayer bending and tilt variation along the monolayer surface. In monolayers with vanishing tilt, these variables are the same as J and K, respectively, in Eq. 1. The values of km, Js, and κ in Eq. 2 are assumed to have the same values as in Eq. 1, and these values are as measured via experiments on HII and QII phases (see below). Thus, in the limit of zero tilt, Eq. 2 reduces to Eq. 1. A tilt model is not necessary to describe the curvature energies of catenoidal-fusion pore structures, because they lack hydrophobic interstices.
are the splay and saddle splay, respectively, which include additive contributions from monolayer bending and tilt variation along the monolayer surface. In monolayers with vanishing tilt, these variables are the same as J and K, respectively, in Eq. 1. The values of km, Js, and κ in Eq. 2 are assumed to have the same values as in Eq. 1, and these values are as measured via experiments on HII and QII phases (see below). Thus, in the limit of zero tilt, Eq. 2 reduces to Eq. 1. A tilt model is not necessary to describe the curvature energies of catenoidal-fusion pore structures, because they lack hydrophobic interstices.
The curvature free energy of a fusion intermediate is the integral of Eq. 1 or Eq. 2 over the area of all the monolayer segments composing the structure. Let F be the total curvature energy of a segment of monolayer. F is the integral of f or ft over the area, A, of a monolayer segment:
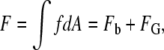 |
(3) |
where FB is the contribution of bending elastic energy and FG is the contribution of Gaussian curvature elastic energy:
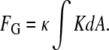 |
(4) |
For the stalk, FG and FB are the area integrals of the second term, and of the sum of the other two terms, respectively, in Eq.2. For flat bilayers, K = 0 and FG = 0.
What is FG for the intermediates in membrane fusion?
For monolayers with smooth interfaces, the change in total area-integrated Gaussian curvature during fusion intermediate formation is determined by the change in topology of the system, including all the lipid monolayers (18). Formation of each stalk between the proximal monolayers of two liposomes changes the area integral of the Gaussian curvature of all the monolayers by −4π. Conversion of each stalk to a catenoidal fusion pore changes the total integrated Gaussian curvature by another unit of −4π.
However, the change in Gaussian curvature energy is determined by the change in the product of κ and the area integral of K for all monolayers in intermediate compositions (Eq. 4). κ is composition-dependent, and the composition can vary from place to place in a biomembrane, so the intermediate energy must be calculated with the local value of κ in each monolayer that forms the intermediate. Let κproximal and κdistal be the values of κ for the lipids in the proximal and distal monolayers, respectively. It is convenient to consider the case of intermediates forming between infinite flat sheets of membrane, since the original flat sheets have K = 0 and thus zero Gaussian curvature energy. For the proximal monolayer of a stalk, ∫KdA = −4π. The Gaussian curvature elastic energy of the stalk, 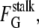 is the sum of the contributions from the proximal and distal monolayers:
is the sum of the contributions from the proximal and distal monolayers:
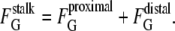 |
(5) |
The integral ∫KdA for the distal monolayers of the stalk is 0. Thus, by Eq. 5,
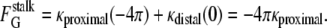 |
(6) |
HFs (3) are radially expanded stalks with a patch of flat bilayer inserted in the center (Fig. 1 B). Since the flat bilayer has zero Gaussian curvature, the FG values for the proximal and distal monolayers are the same as in the stalk:
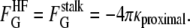 |
(7) |
In the catenoidal fusion pore, the two planar distal monolayers of the original bilayers become continuous, as do the facing monolayers. If we assume that the proximal and distal monolayers have the same value of κ, then, by Eq. 4, the ∫KdA contribution of the distal monolayer is the same as for the proximal monolayer in the stalk:
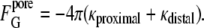 |
(8) |
For monolayers with nonsmooth surfaces, like the proximal monolayer of the stalk in previous studies (2–4,11), tilt contributions to K can change the area-integrated value of K from the value in Eq. 6. However, at least in the case of Kozlovsky et al. (11), this makes only a small difference from the smooth monolayer value in Eq. 6: the Gaussian curvature energy of stalks is −11.8κ, which is only a 6% difference from −4π.
To what extent have values of κ been measured?
Recent measurements of the ratio κ/km in symmetric bilayers, which are accurate to within ∼10%, have been obtained in only two lipid systems: glycerolmonooleate/DOPC/DOPE = 0.58:0.38:0.04, where the ratio is κ/km = −0.75 ± 0.08 (17); and DOPE-Me (18,19), where the ratio is −0.83 ± 0.08 (18) or −0.90 ± 0.09 (19). The method used by Siegel (19) is slightly more accurate than that used by Siegel and Kozlov (18), for reasons discussed by Siegel and Tenchov (19,28). km was assumed to be 10 kBT for DOPE-Me (18), so κ is ∼−9 kBT for this lipid. As will be shown in the course of this work, κ is within <16% of the DOPE-Me value for at least three other lipid systems (DOPC, DOPE, and an equimolar DOPC/cholesterol mixture). It has been argued that for bilayer-forming lipids in general, κ/km must be >−1 (17) and <−0.5 (29). km for many biologically relevant lipid systems is on the order of 10 kBT (29), which suggests that κ is usually in the range −5 to −10 kBT. Therefore, assuming that the value of κ for the four lipids discussed in this work (−8 to −9 kBT) is representative of most membrane lipids, we see from Eqs. 6–8 that the Gaussian curvature energy of fusion intermediates could range between 100 and 200 kBT, which is obviously a very significant contribution. However, as discussed below, contributions of this magnitude are necessary to resolve paradoxes concerning lipid phase behavior that occur if κ is assumed to be 0, as in some previous calculations (1–4,14,25).
Estimating κ on the basis of observed lipid phase behavior
Lipid compositions with symmetric bilayers should form thermodynically stable catenoidal fusion pores and bicontinuous QII phases when the following inequality is satisfied (19):
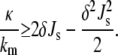 |
(9) |
δ is the distance between the bilayer midplanes and the neutral plane of the lipid monolayers. It is assumed that the neutral plane is at the interface between the hydrophilic and hydrophobic regions of the monolayer. Using data from Rand and Parsegian (30) for monomethylated egg PE, δ is estimated to be 1.3 ± 0.1 nm. Using data from detailed structural studies of the DOPC Lα phase at 30°C (31), one obtains nearly the same value (1.36 nm). Hence, a value of 1.3 ± 0.1 nm is used here for dioleoyl-chain lipids and lipid compositions with similar average chain lengths.
The relationship in Eq. 9 is also applicable to lipid mixtures. If a lipid composition forms bicontinuous QII phases at equilibrium under given conditions, and one knows the values of km, Js, and δ for the lipid composition of the QII phase, one can calculate a lower bound to the value of κ/km via Eq. 9. Conversely, if a QII phase is not observed, then Eq. 9 provides an upper bound to the value of κ/km. Js of the lipid components decreases with increasing temperature. The temperature at which the curvature energies of the Lα and QII phases are equal is denoted as TK (19), and is defined as the temperature at which the equality in Eq. 9 is satisfied. The value of κ/km at T = TK is designated M:
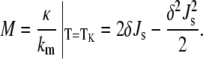 |
(10) |
The value of Js can be measured by x-ray diffraction experiments on samples of the HII phase of the lipid composition in the presence of long-chain alkanes (32). Knowing the values of Js, km, and δ at TK, one can determine the value of κ for the mixture. If we have expressions for the elastic constants of the mixture in terms of the elastic constants of the pure components, we can use Eq. 10 to estimate the Gaussian curvature elastic moduli of lipids that do not form QII phases by themselves. The values of κ and km are expected to change with temperature, but the ratio κ/km for DOPE-Me appears to be constant to within ∼1% across a temperature interval of 35°C (19). The value of km for DOPE decreases linearly with increasing temperature by ∼15% between 10°C and 60°C (33). Hence, the measured values of km and κ are fairly constant across small temperature intervals of a few tens of degrees.
We will only consider mixtures of two lipids for which δ is constant. Let the subscript α indicate the elastic constants of the “host” lipid. The subscript β indicates the elastic constants of the “guest” component, which is a small fraction of the total lipid. The Js of the mixture may be expressed as the molecular-area-weighted sum of the spontaneous curvatures of the pure lipid constituents (Jα and Jβ) (34):
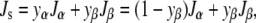 |
(11) |
where the area fractions yα and yβ can be calculated based on knowledge of the area/molecule of each of the two lipids at the neutral plane (aα and aβ, respectively) and their mole fractions (1 − xβ and xβ, respectively):
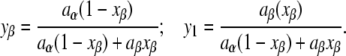 |
(12) |
The spontaneous curvature of binary lipid mixtures is often observed to be approximated by a mole-fraction-weighted sum of the spontaneous curvatures of the lipids (e.g., (35–39)). Equation 12 is a mole-fraction-weighted sum if the two lipid species have equal areas at the neutral plane (aα = aβ). The aα ≈ aβ condition is met by most of the lipids studied in (35–38). The Js of a mixture may also seem linear in the mole fraction of β if aα ≠ aβ, but the area fraction of β is kept small (37,39). Here, we will be concerned predominantly with DOPE and DOPC, which have similar values of area/molecule at the neutral plane (37), so xβ ≈ yβ (to within a few percent).
The bending elastic modulus of a two-component lipid mixture, kmix, is given by (34)
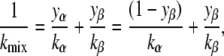 |
(13) |
where kα and kβ are the bending moduli of the pure components. Finally, we need an expression for the monolayer Gaussian curvature elastic modulus of a two-component mixture in terms of the Gaussian moduli of the components. If one adds a term for the Gaussian curvature elastic energy to the expression for the free energy of a lipid monolayer, and performs the same analysis as in Kozlov and Helfrich (34), then the expression for the Gaussian curvature elastic modulus of the mixture is
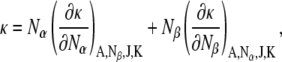 |
(14) |
where Nα and Nβ are the number of molecules of species α and β per unit area, respectively; and A is the monolayer area. We assume that the contributions of the individual lipid species to κ do not depend on the lipid composition (ideal solution behavior). This is a good assumption for weakly interacting lipids, like PE and PC, but might be violated in mixtures with nonideal mixing behavior, like PC and cholesterol. With this assumption, for constant A and constant numbers of lipid molecules Nα and Nβ,
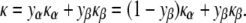 |
(15) |
Expressing M for the two-component mixture (Eq. 10) in terms of Eqs. 11–15, rearranging and retaining terms only to the first power in yβ (since yβ ≪ 1), one obtains
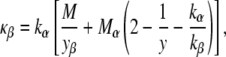 |
(16) |
where Mα = κα/kα, the “host” lipid property that is measured directly by the method in the studies by Siegel and Kozlov (18) and Siegel and Kozlov (19).
THEORETICAL RESULTS
The Gaussian curvature elastic modulus of DOPE, κDOPE
A good estimate of κDOPE can be made on the basis of observed phase behavior. DOPE and DOPE-Me should have similar elastic constant values: the values of km for the two lipids are similar (18,19), and the HII tube diameters at TH are about the same (40,41). On these grounds, one would expect DOPE to form a QII phase upon heating, just as in DOPE-Me (20). This is not observed. Instead, DOPE in excess water forms HII phases if heated through the lamellar (Lα)/inverted hexagonal (HII) phase transition temperature (TH), and only forms QII phases if the temperature is cycled back and forth across TH, between −5°C and 15°C (42,43). Other pure PE systems behave similarly (44,45). This QII phase metastability in PE occurs because of the influence of the particularly strong attraction between Lα phase bilayers in pure PEs as compared to PE-PC mixtures or mono-methylated PEs. The energy of this interaction is absent in the QII phase, where bilayers are several times farther apart than in the Lα phase (28). This effect increases the Lα/QII phase transition temperature (TQ) to be ≥TH. However, the QII phase can form during temperature cycling: each time the system is cooled through TH, most of the HII phase lipid reverts to Lα phase, but a fraction enters the QII phase instead, and the QII phase is easily supercooled once it forms and also persists to temperatures >TH on reheating (20).
The total free energy of lipid in the QII and HII phases, composed of curvature energy and bilayer-bilayer interaction energy contributions (28), must be equal at some temperature within the cycling interval. This condition is expressed as
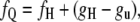 |
(17) |
where fQ and fH are the curvature free energies of the QII and HII phases, respectively, with respect to planar bilayers, and gu is the bilayer-bilayer interaction energy in the Lα phase. gu is a large positive value for DOPE: work must be done to separate the bilayers in the Lα phase so that the QII phase can form. The lipid interfaces in the HII phase of DOPE in excess water are two or three times farther apart than in the Lα phase, but there may be an interaction energy in this phase, gH, whose form is not known. We presume that gH is positive as well, but smaller than gu, so that 0 ≥ gH − gu ≥ −gu.
Since the QII phase does not form before the HII phase on slow heating, the temperature at which the condition in Eq. 17 is met must be <TH, which is 3°C for DOPE (46). At TH, fH = 0, so at this temperature, the value of fQ lies between two values: if gH − gu = 0, fQ has its maximum value of 0, which means that κ/km has the value given by Eq. 10. If gH – gu < 0, then fQ < 0 and TK is at a lower temperature than TH. At the lower temperature of the cycling range, −5°C, the temperature is <TH, and fH is >0. fH can be calculated at a temperature T in terms of the spontaneous curvature at T and TH: Js(T) and Js(TH) (18,47):
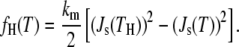 |
(18) |
We assume that Js is equal to the the inverse of the pivotal plane radius (32). This radius has been measured in DOPE at only a few temperatures, mostly at or above room temperature (32,33,36,37,48). A consensus value of 2.85 nm at 22°C (36,37,48) is used. The radius decreases linearly with increasing temperature at a rate that is approximately −0.0109 nm/°C (33). With these values, the extrapolated values of Js at −5°C and TH are −0.318 nm−1 and −0.320 nm−1, respectively. km for DOPE is 9 kBT when corrected for the discrepancy between the neutral and pivotal planes (36). Thus, at −5°C, fH = 0.0261 kBT/nm2. gu has been measured for egg PE and Lα phase POPE as 0.14 erg/cm2 or 0.017 kBT/nm2 (30). With these values, it is clear that fQ at −5°C is >0 for the entire range of values of gH – gu considered here: it does not matter what the exact value of gH is. According to the form of expression of the curvature energy of QII phases (18), if fQ > 0 at a given temperature, then that temperature is <TK, and for DOPE, −5°C < TK ≤ 3°C. Then, according to Eq. 10, M is in the range −0.941 to −0.912, and a value of −0.927 ± 0.015 is used here. The standard errors in the values of the bending elastic moduli are assumed to be ±10%, which is approximately the uncertainty cited in other studies (36,37,48); the errors in the spontaneous curvatures are ±3% (corresponding to an error in neutral plane placement of ±0.1 nm for lipids like DOPE and DOPE-Me, which is a typical error in such x-ray diffraction experiments); and the error in δ is ±0.1 nm. The error in δ is estimated from the difference in values obtained for monomethylated egg PE and DOPC near room temperature (30,31). Therefore, according to Eq. 10, κDOPE = −8.3 kBT ± 1 kBT, where the uncertainty arises from the uncertainty in δ, km, and Js(TK).
The Gaussian curvature elastic modulus of DOPC, κDOPC
κDOPC can be determined from the phase behavior of DOPE/DOPC mixtures using Eqs. 10–16 and the value of κDOPE estimated above. Ideally, one would determine the value of M of a DOPE/DOPC mixture by measuring the temperature dependence of the unit cell constant of the QII phase (19). However, a good estimate can also be derived from the temperature at which isotropic 31P NMR resonances are observed. In a dispersion of large multilamellar liposomes of phospholipids, isotropic 31P NMR resonances arise from QII phases, or the catenoidal fusion pores that are QII phase precursors (18,19). This correspondence of the appearance of isotropic 31P-NMR resonances with QII phase formation has been verifed via x-ray diffraction for DOPE-Me (20,40,49), DEPE and DOPE exposed to temperature-cycling protocols (42–45), and soy PE/egg PC (50). As a function of increasing temperature, the curvature energy of catenoidal fusion pores first becomes equal to that of the Lα phase at TK. Hence, the temperature at which the isotropic component becomes dominant in the 31P NMR spectrum of a phospholipid mixture is an estimate of TK.
The relationship between the temperature at which isotropic 31P NMR resonances appear and TK is approximate, however. The curvature energy of catenoidal fusion pores is small and changes slowly with temperature at temperatures near TK (18,19), so that some of these structures form at temperatures a few degrees below TK. For example, isotropic resonances in DOPE-Me dispersions have been observed starting at temperatures between 10° below TK (49,51–53) and TK (54–56), where TK was later determined via x-ray diffraction (19,20).
31P NMR spectra of 4:1 (mol/mol) mixtures of DOPE/DOPC show an isotropic component at 35°C (49), and the spectrum is almost completely isotropic after prolonged incubation at 40°C (57). The Js and km of DOPC were measured as −0.115 nm−1 and 9 kBT, respectively, at 32°C (37). Thus, 40°C is an estimate of TK for the lipid mixture, and the area/molecule values of DOPE and DOPC at the neutral plane are assumed to be equal. The difference between Js of DOPC between 32 and 40°C is negligible. The value of Js for DOPE at 40°C is estimated to be −0.377 nm−1. For y = 0.2 and kDOPE = kDOPC, Eq. 16 reduces to the simpler form κDOPC = kDOPE(5M − 4MDOPE). The value of κDOPE calculated above is used to calculate MDOPE (Eq. 10). When the relative standard errors assumed are the same as in the calculation of κDOPE, with Eqs. 10–16, one obtains κDOPC = −7.6 ± 1.5 kBT.
The Gaussian curvature elastic modulus of DOPC/cholesterol = 1:1, κPC-CH
It was recently shown that an equimolar mixture of DOPC and cholesterol forms a QII phase in excess water starting at ∼65°C (58). As noted by Tenchov et al. (58), 65°C is an upper bound to TK. The Js values for DOPC/cholesterol mixtures were measured at 32°C in Chen and Rand (37) by adding tetradecane to induce an HII phase in the DOPC/cholesterol mixtures, and then measuring the change in the HII phase unit-cell dimension under osmotic stress (32). If we use Chen and Rand's (37) data to approximate Js as the inverse of the pivot plane radius in the HII phase in the DOPC/cholesterol = 1:1 system, we find that at 32°C, Js = −0.25 nm−1. km for this mixture is 11 kBT (37). Since Js decreases with increasing temperature, Js for the system at 32°C will be smaller (more negative) than the value at 65°C. If the TK of the mixture is 65°C and the temperature dependence of Js for the DOPC/cholesterol mixture is the same as for DOPE-Me (18), this would make the value of Js 16% smaller, or −0.29 nm−1, at 65°C. Therefore, we use a value of Js at TK = −0.27 ± 0.02 nm−1. Assuming the same uncertainties in km and δ as above, with Eq. 10, one obtains κPC-CH = −8.4 ± 1.1 kBT.
The estimated values of κ of the three representative lipid systems are given in Table 1, along with the values of km and Js for T = 32°C.
TABLE 1.
Curvature elastic constants for the lipid compositions
Effect of Gaussian curvature elastic energy contributions on fusion intermediate energies
The discussion here will concentrate on the model for stalk energies of Kozlovsky et al. (11), because more analytical expressions for the dependence of stalk curvature energy on curvature elastic constants are given in that case than in the study by May (4). However, as will be shown below, addition of a Gaussian curvature elastic energy to the energy reported in the study by May (4) will make the stalk energy comparable to or larger than the similarly adjusted values predicted by the theories in other studies (1–3).
The curvature elastic energy of a stalk in excess water, Fs, according to the model of Kozlovsky et al. (11) is given by
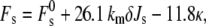 |
(19) |
where 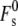 is the splay elastic energy necessary to create a stalk in a lipid system with Js = 0, which is a constant equal to ∼81 kBT. This value of
is the splay elastic energy necessary to create a stalk in a lipid system with Js = 0, which is a constant equal to ∼81 kBT. This value of 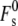 is appropriate for stalks forming between the membranes of two isolated vesicular membranes, as in biomembrane fusion or the fusion of small liposomes, where the membranes around the periphery of the stalk are separated by a distance of >3 nm (11).
is appropriate for stalks forming between the membranes of two isolated vesicular membranes, as in biomembrane fusion or the fusion of small liposomes, where the membranes around the periphery of the stalk are separated by a distance of >3 nm (11). 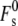 is larger if this distance is smaller: for example, if the interbilayer separation is 2 nm,
is larger if this distance is smaller: for example, if the interbilayer separation is 2 nm, 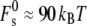 (11). In Kozlovsky et al. (11) the contributions to the curvature elastic energy are due to splay (a combination of monolayer bending and gradients in lipid molecule tilt) and saddle splay (Gaussian) curvature elastic energy. These are given by the sum of the first two terms on the righthand side of Eq. 19, and the last term, respectively. The contribution of tilt elastic energy (the last term in Eq. 2) is contained within the value of
(11). In Kozlovsky et al. (11) the contributions to the curvature elastic energy are due to splay (a combination of monolayer bending and gradients in lipid molecule tilt) and saddle splay (Gaussian) curvature elastic energy. These are given by the sum of the first two terms on the righthand side of Eq. 19, and the last term, respectively. The contribution of tilt elastic energy (the last term in Eq. 2) is contained within the value of 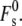
Next, the curvature elastic energy of the catenoidal fusion pore is evaluated. In Siegel and Kozlov (18), it was shown that fusion pores will have catenoidal profiles for membranes in which the two monolayers have the same composition. The catenoid is a surface with zero mean curvature. The catenoidal surface is the bilayer midplane. The curvature energy of the monolayers making up the catenoidal fusion pore is not zero, however, because the neutral planes of the monolayers do not lie on the catenoid surface, but lie on surfaces that are displaced from the bilayer midplanes, and these surfaces have nonzero curvature energy. A model has been developed for the curvature energy of catenoidal fusion pores and QII phases that is accurate to the fourth order in curvature (19). However, here the fusion pore energy is calculated using the second-order curvature energy model in Siegel and Kozlov (18) to be consistent with the second-order calculation for stalks (Eq. 19). For symmetric bilayers, the curvature elastic energy of a catenoidal fusion pore is given by
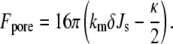 |
(20) |
The contribution of Gaussian curvature elastic energy is −8πκ (Eq. 8). If a catenoidal fusion pore forms between flat bilayers, there must be a region with nonvanishing curvature around the periphery of the pore, which will also contribute to the curvature energy. However, these contributions are small compared to kBT for big enough patches of fusing membranes (59). Moreover, if the catenoidal fusion pore forms between two bilayer membranes that each have even slight nonzero convex curvature, as in the fusion of an intracellular secretory vesicle with a slightly invaginated segment of a cellular plasma membrane, or the fusion of two unilamellar liposomes, then there is no such additional contribution to Fpore; there will generally be a finite radius from the axis of the catenoidal fusion pore at which the tangent angle of the pore membrane is equal to the tangent angle of the surrounding liposomal membrane, as long as the pore radius is smaller than the vesicle radius. Hence, the contribution from the peripheral regions can be neglected in Eq. 20.
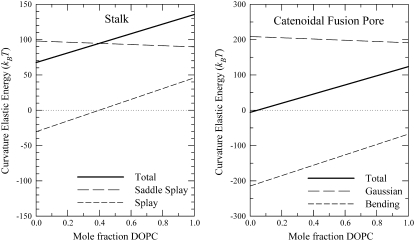
The total curvature elastic energy of stalks and catenoidal fusion pores, along with the contributions from the splay and saddle splay terms for stalks, and the bending and Gaussian curvature elastic terms for catenoidal fusion pores, can be calculated with the values of the elastic constants in Table 1, using Eqs. 8, 19, and 20. These values are displayed in Table 2. The uncertainties in the energies given in Table 2 are only the uncertainties introduced by the uncertainties in the values of κ for each lipid composition (Table 1). They do not take uncertainty in the values of any other variables into account. The intermediate curvature energies can also be calculated for mixtures of DOPE and DOPC using the elastic constants for each of the pure lipids and Eqs. 11–13 and 15. The results of these calculations are displayed in Fig. 2.
TABLE 2.
The curvature elastic energy of stalks and fusion pores (in kBT)
| Stalk
|
Fusion pore
|
|||||
|---|---|---|---|---|---|---|
| System | Saddle splay | Splay | Total | Gaussian | Bending | Total |
| DOPE | 98 ± 12 | −30 | 68 ± 12 | 209 ± 25 | −215 | −6 ± 26 |
| DOPC | 90 ± 18 | 46 | 136 ± 18 | 191 ± 38 | −68 | 123 ± 38 |
| DOPC/cholesterol = 1:1 mol/mol | 99 ± 13 | −12 | 87 ± 13 | 211 ± 27 | −180 | 31 ± 27 |
Values were calculated using the elastic constant values in Table 1. Stalk splay and saddle splay curvature energies were calculated using Eq. 19. The splay and saddle splay elastic energies of stalks are the sum of the first two terms, and the last term, respectively, in Eq. 19. Fusion pore Gaussian curvature elastic energies were calculated using Eq. 8; and the total curvature energies using Eq. 20. The indicated uncertainties in total energy are the uncertainties introduced by the uncertainty in κ for each system (Table 1).
FIGURE 2.
Plot of the curvature elastic energy of stalks (left) and catenoidal fusion pores (right) in DOPE-DOPC mixtures as a function of the mole fraction of DOPC in the mixture. Solid lines represent the total curvature elastic energy, long-dashed lines the splay elastic or bending elastic energy for stalk or catenoidal fusion pore, respectively, and short-dashed lines the saddle splay or Gaussian curvature elastic energy for stalk or catenoidal fusion pore, respectively.
The bending energy values for stalks in Table 2 correspond to the curvature energies one would calculate with κ = 0. The dependence of the stalk bending energies on Js is similar to the dependence of the stalk energies found via the model of May (4), which were also calculated with κ = 0. However, the energies in that study (4) are higher for small Js and lower at Js values closer to 0. The stalk bending energy for the Js of DOPE would be ∼0 (Fig. 7 in May (4)), and for DOPC (Js = −0.115 nm−1) ∼20 kBT. These values are ∼30 kBT higher and ∼26 kBT lower, respectively than the values for DOPE and DOPC in Table 2 of this article. A Gaussian curvature elastic contribution must be added to the energies calculated by May (4). This contribution would be ∼100 kBT for both lipids (Eq. 6). With this contribution, the total stalk energy for the May model (4) would be ∼100 kBT for DOPE and 120 kBT for DOPC. However, these are approximate comparisons. A smaller value, km = 6.8 kBT, was used by May (4), and a different energy optimization procedure was applied than in Kozlovsky et al. (11). For example, the monolayer thickness was taken as another degree of freedom in May (4). To better compare the two models, the stalk energies should be recalculated using the same values for the elastic constants, the values of κ estimated here, and the optimization procedure described in May (4).
DISCUSSION
The values of κ for the different lipid compositions are similar (Table 1), and similar to the value measured for N-monomethylated DOPE (19), despite the large uncertainty in the calculations. This may not be surprising in light of the similarity in the values of km for the same lipids. The Gaussian curvature elastic energies for stalks and catenoidal fusion pores are large and positive for all the lipid compositions (Table 2, Fig. 2), and are frequently larger in absolute magnitude than the contributions from bending or splay elastic energy.
The data in Fig. 2 also show that intermediate energies that include the Gaussian (saddle splay) curvature elastic contribution are consistent with observed lipid phase behavior in excess water. First, the total stalk curvature energies are >0 for DOPE, DOPC, and their mixtures (Table 2 and Fig. 2). This is compatible with the absence of any rhombohedral phases in excess water in these compositions (60). In contrast, if κ = 0 in Eq. 19, as in Kozlovsky and Kozlov (2), and the only contribution to the total curvature elastic energy is from splay, then Eq. 19 would predict the existence of thermodynamically stable stalks in excess water in DOPE-DOPC mixtures near room temperature for mixtures with <40 mol % DOPC (Fig. 2 left, long-dashed line). Rhombohedral phases composed of stalks are observed in DOPC and in DOPE-DOPC mixtures (60), but not in pure DOPE, and only if the lipids are extensively dehydrated to water activities of 0.4–0.8. Extensive dehydration lowers the amount of energy necessary to create stalks from the Lα phase: dehydration forces bilayers into closer proximity against strong repulsive forces, whereas rhombohedral phase (stalk) creation from the Lα phase destroys the bilayer/bilayer interface (11).
Second, the total curvature energy of catenoidal fusion pores is close to zero for DOPE and for mixtures of DOPE with small mole fractions of DOPC (Table 2 and Fig. 2). This is compatible with the isotropic 31P NMR resonance observed for DOPE-rich mixtures of these lipids at 40°C, close to the temperature used for our calculations (49). The catenoidal fusion pore energy is also low for DOPC/cholesterol = 1:1 (Table 2), which is compatible with the formation of a QII phase at a higher temperature (∼65°C (58)). In Fig. 2, the total curvature energy of a catenoidal fusion pore increases as the mole fraction of DOPC increases in a DOPE-DOPC mixture, consistent with the observation that the temperatures at which QII phases and QII phase precursors appear increase with increasing mole fraction of DOPC in DOPE (49). In contrast, if κ = 0 in Eqs. 10 and 20, then catenoidal fusion pores would form a thermodynamically stable phase in all the lipid compositions treated in this work, and in fact in any lipid composition with Js < 0 (like pure DOPC), which is not observed.
Comparison of predicted stalk energy with the rates of stalk-mediated processes in pure lipid systems
The curvature energies must not make the activation energy for formation of fusion intermediates so high that they cannot form at the rates of observed fusion processes. Lipid mixing and fusion between lipid vesicles are examples of processes that are thought to be stalk-mediated. In all the cases in Table 2 and Fig. 2, the curvature energy of the catenoidal fusion pores is less than the curvature energy of the stalks. This suggests that the energy barrier to stalk creation is the primary barrier to fusion in pure lipid systems. To minimize the number of assumptions about the kinetic scheme for interconversion of fusion intermediates (e.g., reversible versus irreversible reactions, existence of side reactions, etc.), rate estimations will only be made for stalks.
To make order-of-magnitude estimates of the effects of intermediate energy on the rate of stalk-mediated processes without detailed knowledge of the kinetic scheme for intermediate formation, further simplifying assumptions are necessary. First, we assume that formation of the intermediate is a simple one-step process. In reality, processes like close opposition of two membranes may have to precede formation of a stalk structure between them. However, it was recently proposed that the membranes first come into contact via a stalklike deformation of one of the opposed membranes, and that the energy of this deformation is lower than that of the resulting stalk (61). Hence, the membrane-membrane contact and intermediate formation step might be treatable as a single process.
Second, it is assumed that the total curvature elastic energy of the stalk with respect to flat bilayers is a lower-bound estimate to the total activation energy for formation of the intermediate, W. With these assumptions, we can use an equation for estimating the order-of-magnitude waiting time, τ, for formation of stalk intermediates that was proposed by Weaver and Mintzer (62) and Kuzmin et al. (25):
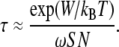 |
(21) |
S is the area/fusion site, and N is the number of possible fusion sites. ω is the characteristic frequency of fluctuations within lipid monolayers, which is taken to be 1011 s−1 nm−2 (62). We consider the area of interaction between a unilamellar liposome with a radius of 50 nm and another membrane. It is assumed that the area of membrane-membrane contact is a disk with diameter equal to one liposome radius, so the contact area is π(50 nm)2. The area of interface that can form a stalk is π(3 nm)2, so the number of possible stalk initiation sites is (50 nm)2/(3 nm)2, and SN = 8 × 103 nm2. It is emphasized that Eq. 21 is only intended to yield estimates that are accurate to within a few orders of magnitude.
First, let us consider the rates of processes in protein-free lipid systems. Time-resolved cryoelectron microscopy experiments have shown that large unilamellar vesicles (LUVs) composed of egg PE or DOPE-Me fuse extensively within 16 ms when subjected to a temperature jump to T > TH (21). Using W = 68 kBT for pure DOPE (Table 2), we find τ ∼ 1014 s for stalk formation. The stalk energy for DOPE in Table 2, and hence W, has an uncertainty due to uncertainty in κ of 12 kBT, which makes τ uncertain to within a very large factor of 105. Even so, a figure of τ ∼ 1014 s indicates that the model for the curvature energy of stalks in Kozlovsky et al. (11) yields values that are obviously too large to be compatible with the observations in Siegel et al. (24). The estimated value of τ suggests that the model used here (11) overestimates the stalk curvature energy by ∼30 kBT or more.
The Lα/HII transition in the bulk Lα phase is also thought to begin with stalk formation (3,14,63). Significant numbers of stalks must form on timescales shorter than the half-time of the transition. In PE Lα phases, in which the water/bilayer interfaces are ∼1–1.5 nm apart (30), the appropriate value for formation of stalks (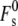 in Eq. 19) is ∼95 kBT (11). This value of
in Eq. 19) is ∼95 kBT (11). This value of 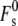 includes the effect of constraints on the shape of the stalk due to repulsive forces between adjacent membranes in the multilayer stack. With this value of
includes the effect of constraints on the shape of the stalk due to repulsive forces between adjacent membranes in the multilayer stack. With this value of 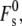 the total energy of stalks forming within a bulk Lα phase of DOPE would be ∼82 kBT (Eq. 16). A 10-μm cube of Lα phase (chosen to represent an average multilamellar liposome) contains ∼8 × 109 fusion sites if we use the same value of S as before. With these values, Eq. 21 predicts a waiting time for formation of even a single stalk in an MLV of 1015 s. Even with an uncertainty in W of 12 kBT, this very long waiting time is incompatible with measurements of the Lα/HII transition rate in DOPE MLVs. The Lα/HII transition has a half-completion time of several tens of seconds or less when a sample is superheated by ≥5° (64), and ∼1 s when samples are superheated by 30° (65). This discrepancy suggests that the stalk curvature energy estimated by the model in Kozlovsky et al. (11) is too high by >30 kBT; a result similar to our estimate for fusion in unilamellar liposomal systems. The stalk model in May (4) seems to generate an even higher total stalk curvature energy for the DOPE system, but as noted in the Theory section, more detailed calculations would be required to test this.
the total energy of stalks forming within a bulk Lα phase of DOPE would be ∼82 kBT (Eq. 16). A 10-μm cube of Lα phase (chosen to represent an average multilamellar liposome) contains ∼8 × 109 fusion sites if we use the same value of S as before. With these values, Eq. 21 predicts a waiting time for formation of even a single stalk in an MLV of 1015 s. Even with an uncertainty in W of 12 kBT, this very long waiting time is incompatible with measurements of the Lα/HII transition rate in DOPE MLVs. The Lα/HII transition has a half-completion time of several tens of seconds or less when a sample is superheated by ≥5° (64), and ∼1 s when samples are superheated by 30° (65). This discrepancy suggests that the stalk curvature energy estimated by the model in Kozlovsky et al. (11) is too high by >30 kBT; a result similar to our estimate for fusion in unilamellar liposomal systems. The stalk model in May (4) seems to generate an even higher total stalk curvature energy for the DOPE system, but as noted in the Theory section, more detailed calculations would be required to test this.
The total stalk curvature energy in DOPC is estimated to be 136 ± 18 kBT (Table 2). With this value of W, τ ∼ 1044 s. According to this estimate, DOPC LUVs should not fuse with each other in excess water. This is certainly compatible with their observed stability in excess water and the fact that they remain stable even if a quite substantial stress is applied via suspension in 10% PEG (66). The PEG concentration required to induce fusion in small unilamellar vesicles composed of PE and PC mixtures increases with increasing PC content (67), as one would expect if the energetic barrier to membrane fusion increased with increasing PC content (Fig. 2). Thus, the energy predicted via Eq. 20 is consistent with observations for DOPC liposome fusion in the sense that DOPC liposome fusion is extremely slow or absent in excess water, and that the energy needed to create a stalk decreases with increasing PE content. The total stalk energies estimated for DOPC according to the May model (4) are also consistent with this conclusion.
In summary, the curvature energies of stalks calculated as in Kozlovsky et al. (11) are too large to be compatible with observed rates of lipid mixing and fusion in LUVs of pure DOPE (24), or with the observed rates of Lα/HII phase transitions in DOPE (64,65). Assuming that vesicle fusion and the Lα/HII transition are stalk-mediated, the model in Kozlovsky et al. (11) seems to overestimate the energy by 30 kBT or more. Similar differences are found for the May theory (4). Discrepancies of this size may be due to such factors as a difference between the estimated versus actual value of kt or to gaps in the theories that have not been identified. It should also be pointed out that the expression for the stalk curvature energy in Eq. 19 contains the difference between a large positive contribution (the first and last terms) and a negative contribution that is similar in absolute magnitude (the second term). Hence, especially for lipids with low Js, like DOPE, part of the apparent discrepancy in total energy may be the result of inaccuracies in the values of the elastic constants in the respective terms. The Kozlovsky et al. theory (11) rationalizes many qualitative and semiquantitative observations of lipid mixing and membrane fusion in pure lipid systems, such as the effects of lipid composition and bilayer composition asymmetry (5–9). It is important to note that the predicted stalk and catenoidal fusion pore energies are now consistent with observed lipid phase behavior, and that the apparent discrepancy in stalk energy estimated here is considerably smaller than those calculated using early stalk models (14), which was later referred to as the “energy crisis” (2). With inclusion of saddle splay and Gaussian curvature elastic energies, models of fusion intermediate energy (11,19) begin to pass the two tests proposed in the Introduction for models of fusion mechanisms. These models are accurate enough to make semiquantitative predictions about how proteins mediate biomembrane fusion. The models might be refined further by more detailed tests of predictions concerning rhombohedral (R) and QII phase stability, and by further consideration of the effects of local variations in lipid monolayer thickness (4).
The intermediate energies in this work were calculated using a continuum theory that was derived for systems with radii of interfacial curvature that are much larger than molecular dimensions (13). The radii of curvature of the stalk monolayers are comparable to or even smaller than lipid molecular dimensions, especially at the “waist” of the stalk (Fig. 1 A). As noted in Kozlovsky and Kozlov (2), the theory in Helfrich (13) successfully predicted the curvature energies in HII phases with similarly small radii of interfacial curvature (36,47,68,69). However, the interface in the HII phase has no Gaussian curvature. There is an apparent discrepancy between the stalk energies calculated in this work and the energies that are consistent with the observed rates of stalk-mediated processes. Perhaps some or all of this discrepancy is due to a failure of the continuum model to correctly estimate the curvature energies of surfaces with large geometric and Gaussian curvatures. One way to test this would be to determine whether models based on the continuum theory (13) accurately describe the stability of rhombohedral phases as a function of water activity, lipid composition, and temperature. A previous theoretical study of rhombohedral phase stability (11) could not unambiguously resolve this issue, in part because the rhombohedral phases in question only form at water activities of 0.4–0.8 (60). As noted in Kozlovsky et al. (11), the elastic constants (km, Js, κ) are determined by separate experiments on systems at near unit water activity. The actual values of these constants may be different in such extensively dehydrated systems. The best test would be to apply the theory in Kozlovsky et al. (11) to rhombohedral phases that form at water activities closer to unity. There is preliminary evidence that some lipid compositions form at least some rhombohedral phase at a water activity of 0.9 (H. W. Huang, Department of Physics and Astronomy, Rice University, 2008, personal communication).
Estimated lipid intermediate energies for biomembrane fusion
Estimating the energy required to form fusion intermediates in a biomembrane lipid composition may give us insight into the mechanism of fusion. If the energy of a pure lipid fusion intermediate is too high for the intermediate to form at rates similar to biomembrane fusion, the discrepancy can be assigned to a difference in fusion mechanism relative to the mechanism corresponding to formation of the pure lipid intermediate; to a discrepancy in the model for calculating the energy of the lipid intermediate; to the effects of fusion proteins on intermediate energy; or to some combination of these possibilities. For the purposes of the calculations here, it is assumed that the stalk-HF-catenoidal fusion pore progression in a patch of membrane lipid is the basis of protein-mediated membrane fusion. We consider three illustrative cases; influenza virus lipid mixing with protein-free liposomes, exocytosis at central nervous system synapses, and fusion of sea urchin cortical granules.
The lipid compositions of the two lipid monolayers of biomembranes are generally different. It is important to estimate the effect of the differences in the elastic constants between the two monolayers on fusion intermediate energy. The expression for the curvature energy of the stalk (Eq. 19) was derived for symmetric-bilayer compositions. However, the splay elastic energy of the stalk is insensitive to the value of Js of the distal monolayer (2), and most of the change in curvature is concentrated in a small area of opposed monolayer area. The opposed monolayers are the only ones that undergo a topological change, and thus the only ones that make a substantial contribution to the change in saddle splay (Eq. 6). Thus, it is a reasonable approximation to use Eq. 19 to estimate the curvature energy of stalks in asymmetric bilayers, using the elastic constants corresponding to the lipid composition of the opposed monolayers. The bending energy of catenoidal fusion pores is sensitive to the elastic constants of both monolayers (1).
Influenza virions have lipid compositions resembling raft microdomains (70). Cholesterol is ∼44 mol % of the membrane. The phospholipid composition is about equally divided between three lipid classes: PE; a combination of PC and sphingomyelin; and acidic lipids (phosphatidylserine and phosphatidylinositiol). More than 60% of the phospholipid acyl chains are saturated (71). Phosphatidylcholine is concentrated in the external leaflet of the virus, as it is in the host cell from which the virus buds (72). The high proportion of saturated acyl chains and the high combined PC and sphingomyelin content probably make both km and Js for the external leaflet of the viral membrane larger than for the DOPC/cholesterol = 1:1 case in Table 2. km is probably greater than for pure DOPC due to the large proportion of saturated acyl chains, but the high cholesterol content could make Js considerably less than for pure DOPC. However, if the viral membrane separates into liquid ordered and disordered phase regions, we cannot be sure which phase is most relevant for fusion activity. Hence, the patches of membrane that actually fuse might be lower in saturated chain lipids and cholesterol than the bulk composition. As for the influence of the external leaflet of the target liposomes, the lipid composition of the target liposomes did not have much effect on the overall rate of lipid mixing under the conditions used in other studies (73–76). In fact, the target membranes were >90 mol % DOPC in many cases (73–76), and the balance was ganglioside or glycophorin, which were added to act as receptors for the viral hemagglutinin. This suggests that the average of the lipid compositions of the viral and target membranes under these circumstances corresponded to a liquid disordered phase (e.g., enriched in DOPC). Therefore, it is assumed that the curvature energies of purely lipidic stalks for the influenza virus/DOPC LUV system lie between the values for DOPC/cholesterol and pure DOPC calculated in Table 2. The typical half-times for lipid mixing reported in other studies (73–76) were tens of seconds. Using the value of SN = 8 × 103 nm2 for interaction of vesicles of radius 0.1 μm, as estimated above, it is seen that to have τ ≈ 1 s in Eq. 21, W must be <37 kBT. The curvature elastic energy of stalks in DOPC/cholesterol = 1:1 is 87 ± 13 kBT, and the value for DOPC is 136 ± 18 kBT. This suggests that influenza-virus-induced lipid mixing could only proceed via lipidic stalk intermediates according to the model in Kozlovsky et al. (11) if the proteins perform functions corresponding to a reduction in W of 50–100 kBT. Note that this estimate for the required effect of proteins on W may be decreased by the discrepancy in predicted versus observed lipidic stalk energy estimated above.
In the case of synaptic vesicle/plasma membrane fusion, the compositions of the external leaflet of the synaptic vesicle membrane and of the synaptic plasma membrane are taken to be similar (77). The synaptic vesicle membrane composition (78) consists of approximately equal weights of cholesterol and phospholipid, with a combination of PE and plasmenyl-PE representing 42 mol % of the phospholipid, PC and sphingomyelin representing 43 mol %, and PS 12 mol %. A large fraction of the acyl chains of the phospholipids are highly unsaturated, especially for the PE fraction. Since the combined fraction of high-curvature lipids (PE, plasmenyl PE, and cholesterol) is high and the acyl chains are extensively unsaturated, and PE is enriched in the external leaflet of synaptic vesicle bilayers (79) and in the cytoplasmic leaflet of plasma membranes, it is possible that the Js for the two opposed monolayers is intermediate between that of pure DOPE and DOPC/cholesterol = 1:1. Synaptic vesicles are ∼40 nm in diameter, and the lengths of the SNARE complex components are of order 10 nm (78), which suggests that there may be only one fusion site available per docked vesicle. In this case, SN ≈ 30 nm2. The time constant for release of the readily-releasable pool of synaptic vesicles is 2–4 ms when Ca2+ influx is not rate-limiting (80,81). Using a waiting time of 1 ms, one finds, via Eq. 21, that W must be <22 kBT. The total curvature energies of stalks in pure DOPE and equimolar DOPC/cholesterol are 68 ± 12 kBT and 87 ± 13 kBT, respectively (Table 2). This suggests that the protein fusion “machinery” has to perform functions corresponding to a reduction in W of 50–70 kBT for fusion to occur on physiological timescales via formation of stalks, as described in Kozlovsky et al. (11). As with influenza hemagglutinin-mediated fusion, this estimate for the required effect of proteins on W may include the apparent discrepancy in lipidic stalk energy estimated here.
Sea urchin cortical vesicles (CVs) undergo fusion in a calcium-dependent manner (82,83). The lipid composition of the membranes of these vesicles (84) is somewhat similar to that of synaptic vesicles (SVs), and CVs and SVs have about the same weight ratio of cholesterol to phospholipid, although in CVs, PE represents a smaller fraction of the phospholipids, and there is almost no sphingomyelin. In addition, CV membranes seem to contain high levels of triacylglycerols (23% of the total lipid), and lower levels of monacylglycerols, free fatty acids, and lyso-PE. It is difficult to assess the influence of these latter compounds. However, we proceed by making the same assumption as for SVs, that the elastic constants for the two opposed monolayers in the CV system are intermediate between those of pure DOPE and DOPC/cholesterol = 1:1. CVs are ∼1 μm in diameter (85), so we assume a contact-area diameter equal to one vesicle radius, and calculate SN in Eq. 21 to be ∼2 × 105 nm2. The initial rate of CV fusion upon exposure to Ca2+ is on the order of 100%/s (83), so τ must be ∼0.1 s. For this value of τ, W must be <35 kBT. This is ∼30 and 50 kBT smaller, respectively, than the total curvature energy of stalks in the pure DOPE and DOPC/cholesterol systems. This estimate is similar to that for SV/plasma membrane fusion, above, except that the fusion-mediating proteins in CV would have to reduce W to a slightly smaller extent for the stalk formation rate to correspond to the observed fusion timescale.
The stalk-pore theory is successful in explaining many qualitative features of biomembrane fusion (5–9), including recent observations of the effects of several exogenous lipids on biomembrane fusion rates (82) and the effects of exogenous cholesterol (83,86). Cholesterol increases the rate of exocytosis in sea urchin cortical granule fusion (83) and of hemifusion in hemagglutinin-induced cell fusion (86), and also promotes fusion pore opening in the latter system. These two roles are consistent with the expected reduction in stalk and HF energy by a lipid with such a negative value of Js (37), and with the observed role of cholesterol in stabilizing catenoidal fusion pores and QII phases (58). It is likely that intermediates resembling stalks mediate biomembrane fusion. However, as estimated above, it appears that the curvature energies of purely lipidic stalks are too high, by 30–100 kBT, to be compatible with the observed rate of stalk-mediated processes in biomembranes. Part of this discrepancy may be due to inaccuracies in calculating the energy of lipidic stalks, since the energies for lipidic stalks, at least for PE, appear to be 30 kBT or more too high. The balance of the apparent discrepancy in stalk energy in biomembrane systems (as much as 70 kBT, depending on the system and the assumptions) must be due to some influence of the fusion-mediating proteins. How can fusion-mediating proteins reduce the energy of the first fusion intermediates in the context of the stalk model?
Can fusion-mediating proteins in vivo increase the rate of formation of lipid fusion intermediates by changing the local curvature elastic constants of lipid monolayers?
Proteins can impose membrane curvature that favors intermediate formation in at least three ways: scaffold creation, local modification of spontaneous curvature, and induction of bilayer asymmetry (see (6,87–89) for reviews). In modification of the local spontaneous curvature, protein moieties bind to the lipid-water interface, insert between the lipid headgroups, and impose a curvature on a small patch of the interface (88). In particular, Martens et al. (90) recently proposed that insertion of synaptotagmin C2 domains into the plasma membrane drives stalk formation by inducing a positive spontaneous curvature in an annulus of monolayer surrounding the monolayer patch that forms the stalk. It is postulated that this dimples the plasma membrane toward the synaptic vesicle membrane, and also places the central patch of monolayer that forms the stalk under positive curvature stress, so that its curvature energy is closer to that of a stalk. In Martens et al. (90), it was estimated that synaptotagmin binding could reduce the stalk energy by ∼20 kBT.
In principle, moieties of fusion-mediating proteins can also bind to the opposed monolayer interfaces that form the stalk. Most of the fusion-mediating machinery is in the cytoplasmic space in synaptic vesicle fusion, and outside of the virions in influenza virus fusion. Moreover, the area of monolayer involved in stalk formation is not large, and few peptide-lipid interactions would be required. The area of opposed monolayer in either membrane that participates in stalk formation is ∼30 nm2, and the monolayer-water interfacial area in the highest-curvature region of a stalk is ∼50 nm2. For example, it is conceivable that several fusion peptides could bind to the monolayers of the fusion patch before stalk formation, or stabilize the interfaces of a nascent stalk. Another possible energy-reducing arrangement may be disposition of positive charges on the surfaces of proteins immediately surrounding the fusion patch. If arrayed in a semitoroidal geometry around the fusion patch, these might interact with negatively charged lipids on the surface monolayer of a nascent stalk, effectively stabilizing the negative curvature of this surface. For catenoidal fusion pores, most of the Gaussian and mean curvature is concentrated in the region within several nanometers of the minimum radius of the pore (19). Protein binding to the lipid-water interface in this region could lower the curvature energy of the nascent catenoidal fusion pore.
Recently, a fusogenic peptide derived from the fusion-mediating protein of the HIV virus was reported to reduce the km of PC membranes by a factor of 3 or more relative to the pure lipid at peptide/lipid molar ratios of only 0.01–0.03, which corresponds to the concentrations expected at fusion sites in vivo (91). The modulus was measured by analyzing the wavelike fluctuations of bilayers in an Lα phase. As noted by Tristram-Nagle and Nagle (91), it is possible that the apparent reduction in km is due to a special arrangement of peptides in the bilayers, so that the reduction might not be the same for deformations such as those found in stalk formation. Ideally, the effect of the peptide on the monolayer bending modulus should be measured via osmotic stress experiments in the HII phase (32), where the curvature of the interface is more similar to the curvature expected in a stalk. However, the data in Tristram-Nagle and Nagle (91) show that fusion-mediating peptides can create a lipid-peptide assembly with drastically different elastic constants than the initial lipid monolayer. In a recent theoretical study, Zemel et al. (92) showed that amphiphilic helical peptides that adsorb to the lipid-water interface strongly reduce the bilayer thickness, but also induce either strong positive or negative bilayer curvature, depending on the depth of insertion of the helix. Peptide binding can also simultaneously increase in bilayer bending elastic modulus by as much as severalfold. Both of these effects occurred at a peptide/lipid ratio of only 0.05. The induced bilayer spontaneous curvature ranged between −0.3 and + 0.1 nm−1. Only rigid cylindrical peptides were treated in Zemel et al. (92), and the peptide effects on the Gaussian modulus were not calculated. Rigid cylinders that interact strongly with the hydrophobic monolayer interior would probably increase the energy necessary to make membrane or monolayer deformations with nonzero K (92). However, this may not be true of anisotropic inclusions like bent or kinked amphipathic helices bound to lipid-water interfaces, which could stabilize a monolayer in a configuration with K < 0 (93,94), effectively lowering the local value of κ.
How would a large peptide-binding-induced change in km, alone, affect the curvature energies of stalks forming from the same patch of opposed membranes? In Eq. 19, 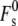 is the splay elastic energy of a stalk when Js = 0, whose value depends on the value of km and the elastic constant for gradients in molecular tilt along lipid interfaces, for which there is no directly determined value (2). No analytical expression for
is the splay elastic energy of a stalk when Js = 0, whose value depends on the value of km and the elastic constant for gradients in molecular tilt along lipid interfaces, for which there is no directly determined value (2). No analytical expression for 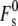 in terms of km and tilt elastic constants was given in the studies by Kozlovsky and co-workers (2,11). If peptide binding to the membranes reduces km as profoundly as the fusion peptide in Tristram-Nagle and Nagle (91), it is likely that the value of
in terms of km and tilt elastic constants was given in the studies by Kozlovsky and co-workers (2,11). If peptide binding to the membranes reduces km as profoundly as the fusion peptide in Tristram-Nagle and Nagle (91), it is likely that the value of 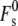 would be decreased. For low-curvature lipid systems like DOPC, this could reduce the stalk curvature energy. However, because Js < 0, Eq. 19 is the sum of the negative term in kmJs and two large and positive contributions, from
would be decreased. For low-curvature lipid systems like DOPC, this could reduce the stalk curvature energy. However, because Js < 0, Eq. 19 is the sum of the negative term in kmJs and two large and positive contributions, from 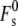 and from the term in κ. For the low-Js systems, DOPE and equimolar DOPC/cholesterol, if peptides profoundly decrease the value of km, the reduction in absolute magnitude in the kmJs term could offset or exceed the effect on
and from the term in κ. For the low-Js systems, DOPE and equimolar DOPC/cholesterol, if peptides profoundly decrease the value of km, the reduction in absolute magnitude in the kmJs term could offset or exceed the effect on 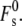 Thus, the effect of a change in km is sensitive to the local value of Js.
Thus, the effect of a change in km is sensitive to the local value of Js.
In contrast, even small peptide-induced changes in κ alone would have large effects on the curvature energies of stalks and catenoidal fusion pores of all lipid compositions. The Gaussian curvature elastic contribution to the total curvature energy is large and positive for stalks and catenoidal fusion pores in all three lipid compositions (Table 2). Increasing κ by 20% (i.e., making it less negative) lowers the total stalk and catenoidal fusion pore energies by ∼20 and 40 kBT, respectively (Table 2). The fusion intermediate energy is especially sensitive to changes in κ, in both low- and high-curvature lipid systems.
However, it is more realistic to consider the effects of peptide-induced changes in combinations of elastic constants. It is unlikely that binding of peptides to the lipid-water interface can change any of the elastic moduli of the lipid monolayers independently of the others. km, Js, and κ are all related to the monolayer stress profile, which is the horizontal force as a function of depth in the plane of the monolayer (15). The first moment of the stress profile along the direction perpendicular to the monolayer is equal to –kmJs, and the second moment to κ (15). Binding peptides to the monolayer-water interface will change the distribution of mass and intermolecular forces as a function of depth, which should have at least some effect on both moments of the stress profile, and hence on all three quantities. Moreover, peptide adsorption to the monolayer can change the bilayer thickness (95) and, hence, δ. The curvature energies of the fusion intermediates are sensitive to relative changes in different elastic constants. The expression for the curvature energy of stalks (Eq. 19) can be rearranged to yield
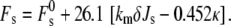 |
(22) |
The term in brackets in Eq. 22 is almost identical to the term in brackets in the expression for Fpore (Eq. 20). Thus, we see that the curvature energies of stalks and of catenoidal fusion pores in symmetric bilayers are both linearly dependent on nearly the same quantity. To determine the effect of a change in lipid-peptide monolayer composition of the fusion patch on fusion intermediate energy, we generally must determine the change that is produced in the quantity (kmδJs − κ/2). For catenoidal fusion pores in asymmetric bilayers like biomembranes, the bending energy component of Fpore is more complicated than in Eq. 20, and will depend on the values of km, Js, and δ for each monolayer, as well as on the catenoidal pore radius. However, the Gaussian curvature elastic contribution to Fpore will be proportional to the sum of the κ values for the two monolayers (Eq. 8). Thus, the expression for Fpore in the asymmetric bilayer case will have a form similar to Eq. 20 in the sense that Fpore will be proportional to the difference between two terms: an expression in terms of bending energy constants, and the sum of Gaussian curvature elastic moduli for the two monolayers.
The two terms inside the brackets in Eqs. 20 and 22 are of similar absolute magnitude, so small simultaneous changes in more than one constant can lead to large changes in total fusion intermediate energy, including large reductions. For example, if peptide-lipid interactions induce a simultaneous 25% increase in κ and 25% decrease in kmδJs in a patch of equimolar DOPC/cholesterol, then the stalk energy decreases by ∼50 kBT relative to the peptide-free monolayer. This would be partially offset by an increase in 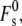 and it is presumed that this increase can be no more than 25% (assuming a direct proportionality of
and it is presumed that this increase can be no more than 25% (assuming a direct proportionality of 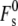 to km), or ∼20 kBT. The net reduction in stalk energy of >30 kBT is comparable to the activation energy reductions required for fusion protein activity that were estimated above.
to km), or ∼20 kBT. The net reduction in stalk energy of >30 kBT is comparable to the activation energy reductions required for fusion protein activity that were estimated above.
The degree to which isolated peptides change the energy of stalks and catenoidal fusion pores can be inferred from the effects of the peptides on the stability of the R and QII phases. The free energy of the stalk-based rhombohedral phase (60) is linearly dependent on (kmδJs − 0.452κ) (11). The quantity (kmδJs − κ/2) is the stability criterion for QII phase to the second order in curvature; QII phases are stable when this quantity is <0 (18). In a sense, preparing samples of these two phases is like preparing large samples of lipidic fusion intermediates in the laboratory. Insight into which elastic constants are most affected by binding of isolated peptides can be obtained by measuring the peptide effects on Js, km, and δ. Js and km can be determined in the presence of peptide by appropriate x-ray diffraction experiments on peptide-lipid-alkane HII phases (32), and δ by x-ray diffraction experiments on peptide-lipid Lα phases. Such studies make the assumption that the peptides are not maintained in some particular orientation to the bilayers by the structure of the other proteins around the fusion site in vivo, however. Some care must be taken in interpreting the results from R phases. So far, R phases have only been observed (60) at low water activities (0.4–0.8), and lipid elastic constants may be different under such dry conditions from those in excess water (11).
The effects of bilayer-spanning peptides may be especially interesting to study more extensively. Peptides corresponding to the bilayer-spanning regions of viral (96,97) and SNARE (98) fusion proteins, as well as synthetic bilayer-spanning peptides (99), accelerate fusion in otherwise protein-free lipid vesicles (96–99). At least some synthetic membrane-spanning peptides have also been shown to lower the temperature at which QII phases form by as much as 20° at peptide/lipid ratios of 0.005 (100). It can be shown that this corresponds to a reduction of 20 kBT in catenoidal pore energy compared to the pure lipid. It is not clear how the peptides affect the free energy of the QII phase (100): if the peptides change one or more monolayer elastic constants, the peptides are at such a low concentration that an individual peptide must somehow be able to influence the elastic behavior on the length scale comparable to the interpeptide separation of >8 nm. If such is the case, particular species of membrane-spanning peptides at the periphery of the fusion patch could also reduce the stalk curvature energy.
Acknowledgments
The author is grateful to M. M. Kozlov for useful discussions.
Editor: Peter Tieleman.
References
- 1.Markin, V. S., and J. P. Albanesi. 2002. Membrane fusion: stalk mechanism revisited. Biophys. J. 82:693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozlovsky, Y., and M. M. Kozlov. 2002. Stalk model of membrane fusion: solution of energy crisis. Biophys. J. 82:882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozlovsky, Y., L. V. Chernomordik, and M. M. Kozlov. 2002. Lipid intermediates in membrane fusion: formation, structure, and decay of the hemifusion diaphragm. Biophys. J. 83:2634–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May, S. 2002. Structure and energy of fusion stalks: the role of membrane edges. Biophys. J. 83:2969–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel, D. P. 2005. Lipid membrane fusion. In The Structure of Biological Membranes, 2nd ed. P. L. Yeagle, editor. CRC Press, Boca Raton, FL. 243–308.
- 6.Chernomordik, L. V., and M. M. Kozlov. 2003. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72:175–207. [DOI] [PubMed] [Google Scholar]
- 7.Chernomordik, L. V., and M. M. Kozlov. 2008. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 15:675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernomordik, L. V., and M. M. Kozlov. 2005. Membrane hemifusion: crossing a chasm in two leaps. Cell. 123:375–382. [DOI] [PubMed] [Google Scholar]
- 9.Chernomordik, L. V., J. Zimmerberg, and M. M. Kozlov. 2006. Membranes of the world unite! J. Cell Biol. 175:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leikin, S. L., M. M. Kozlov, L. V. Chernomordik, V. S. Markin, and Y. A. Chizmadzhev. 1987. Membrane fusion: overcoming of the hydration barrier and local restructuring. J. Theor. Biol. 129:411–425. [DOI] [PubMed] [Google Scholar]
- 11.Kozlovsky, Y., A. Efrat, D. P. Siegel, and M. M. Kozlov. 2004. Stalk phase formation: effects of dehydration and saddle splay modulus. Biophys. J. 87:2508–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel, D. P. 1993. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys. J. 85:2124–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helfrich,W. 1973. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. C. 28:693–703. [DOI] [PubMed] [Google Scholar]
- 14.Siegel, D. P. 1999. The modified stalk mechanism of lamellar-to-inverted phase transitions and its implications for membrane fusion. Biophys. J. 76:291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seddon, J. 1990. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim. Biophys. Acta. 1031:1–69. [DOI] [PubMed] [Google Scholar]
- 16.Shearman, G. C., O. Ces, R. H. Templer, and J. M. Seddon. 2006. Inverse lyotropic phases of lipids and membrane curvature. J. Phys. Condens. Matter. 18:S1105–S1124. [DOI] [PubMed] [Google Scholar]
- 17.Templer, R. H., B. J. Khoo, and J. M. Seddon. 1998. Gaussian curvature modulus of an amphiphile monolayer. Langmuir. 14:7427–7434. [Google Scholar]
- 18.Siegel, D. P., and M. M. Kozlov. 2004. The Gaussian curvature elastic modulus of N-monomethylated dioleoylphosphatidylethanolamine: relevance to membrane fusion and lipid phase behavior. Biophys. J. 67:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel, D. P. 2006. Determining the ratio of the Gaussian curvature and bending elastic moduli of phospholipids from QII phase unit cell dimensions. Biophys. J. 91:608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherezov, V., D. P. Siegel, W. Shaw, S. W. Burgess, and M. C. Caffrey. 2003. The kinetics of non-lamellar phase formation in DOPE-Me: relevance to membrane fusion. J. Membr. Biol. 195:165–182. [DOI] [PubMed] [Google Scholar]
- 21.Lee, J. K., and B. R. Lentz. 1997. Evolution of lipidic structures during model membrane fusion and the relation of this process to cell membrane fusion. Biochemistry. 36:6251–6259. [DOI] [PubMed] [Google Scholar]
- 22.Kasson, P. M., N. W. Kelley, N. Singhal, M. Vrljic, A. T. Brunger, and V. S. Pande. 2003. Ensemble molecular dynamics yields submillisecond kinetics and intermediates of membrane fusion. Proc. Natl. Acad. Sci. USA. 103:11916–11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chanturiya, A., L. V. Chernomordik, and J. Zimmerberg. 1997. Flickering fusion pores comparable with initial exocytotic pores occur in protein-free phospholipid bilayers. Proc. Natl. Acad. Sci. USA. 94:14423–14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel, D. P., W. J. Green, and Y. Talmon. 1994. The mechanism of lamellar-to-inverted hexagonal phase transitions: a study using temperature-jump cryo-electron microscopy. Biophys. J. 66:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzmin, P. I., J. Zimmerberg, Y. A. Chizmadzhev, and F. S. Cohen. 2001. A quantitative model for membrane fusion based on low-energy intermediates. Proc. Natl. Acad. Sci. USA. 98:7235–7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamm, M. and M. M. Kozlov. 2000. Elastic energy of tilt and bending of fluid membranes. Eur. Phys. J. E. 3:323–335. [Google Scholar]
- 27.May, S., Y. Kozlovsky, A. Ben-Shaul, and M. M. Kozlov. 2004. Tilt modulus of a lipid monolayer. Eur. Phys. J. E. 14:299–308. [DOI] [PubMed] [Google Scholar]
- 28.Siegel, D. P., and B. Tenchov. 2008. Influence of the lamellar phase unbinding energy on the relative stability of lamellar and inverted cubic phases. Biophys. J. 94:3987–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh, D. 2006. Elastic curvature constants of lipid monolayers and bilayers. Chem. Phys. Lipids. 144:146–159. [DOI] [PubMed] [Google Scholar]
- 30.Rand, R. P., and V. A. Parsegian. 1989. Hydration forces between phospholipid bilayers. Biochim. Biophys. Acta. 988:351–376. [Google Scholar]
- 31.Nagle, J. F., and S. Tristram-Nagle. 2000. Structure of lipid bilayers. Biochim. Biophys. Acta. 1469:159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rand, R. P., N. L. Fuller, S. M. Gruner, and V. A. Parsegian. 1990. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 29:76–87. [DOI] [PubMed] [Google Scholar]
- 33.Epand, R. M., N. Fuller, and R. P. Rand. 1996. Role of the position of unsaturation on the phase behavior and intrinsic curvature of phosphatidylethanolamines. Biophys. J. 71:1806–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozlov, M. M., and W. Helfrich. 1992. Effects of a cosurfactant on the stretching and bending elasticities of a surfactant monolayer. Langmuir. 8:2792–2797. [Google Scholar]
- 35.Keller, S. L., S. M. Bezrukov, S. M. Gruner, M. W. Tate, I. Vodyanov, and V. A. Parsegian. 1993. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys. J. 65:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leikin, S., M. M. Kozlov, N. L. Fuller, and R. P. Rand. 1996. Measured effects of diacylglycerol on structural and elastic properties of phospholipid membranes. Biophys. J. 71:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen, Z., and R. P. Rand. 1997. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 73:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szule, J. A., N. L. Fuller, and R. P. Rand. 2002. The effects of acyl chain length and saturation of diacylglycerols and phosphatidylcholine on membrane monolayer curvature. Biophys. J. 83:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuller, N. L., and R. P. Rand. 2001. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys. J. 81:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruner, S. M., M. W. Tate, G. L. Kirk, P. T. C. So, D. C. Turner, D. T. Keane, C. P. S. Tilcock, and P. R. Cullis. 1988. X-Ray-diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 27:2853–2866. [DOI] [PubMed] [Google Scholar]
- 41.Tate, M. W., and S. M. Gruner. 1989. Temperature eependence of the structural dimensions of the inverted hexagonal (HII) phase of phosphatidylethanolamine-containing membranes. Biochemistry. 28:4245–4253. [DOI] [PubMed] [Google Scholar]
- 42.Shyamsunder, E., S. M. Gruner, M. W. Tate, D. C. Turner, P. T. C. So, and C. P. S. Tilcock. 1988. Observation of inverted cubic phase in hydrated dioleoylphosphatidylethanolamine membranes. Biochemistry. 27:2332–2336. [DOI] [PubMed] [Google Scholar]
- 43.Erbes, J., C. Czeslik, W. Hahn, R. Winter, M. Rappolt, and G. Rapp. 1994. On the existence of bicontinuous cubic phases in dioleoylphosphatidylethanolamine. Ber. Bunsenges. Phys. Chem. 98:1287–1293. [Google Scholar]
- 44.Veiro, J. A., R. G. Khalifah, and E. S. Rowe. 1990. P-31 nuclear nagnetic resonance studies of the appearance of an isotropic component in dielaidoylphosphatidylethanolamine. Biophys. J. 57:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tenchov, B., R. Koynova, and G. Rapp. 1998. Accelerated formation of cubic phases in phosphatidylethanolamine dispersions. Biophys. J. 75:853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toombes, G. E. S., A. C. Finnefrock, M. W. Tate, and S. M. Gruner. 2002. Determination of the Lα-HII phase transition temperature for 1,2 dioleoyl-sn-glycero-3-phosphatidylethanolamine. Biophys. J. 82:2504–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamm, M., and M. M. Kozlov. 1998. Tilt model of inverted amphiphilic mesophases. Eur. Phys. J. B. 6:519–528. [Google Scholar]
- 48.Chen, Z., and R. P. Rand. 1998. Comparative study of the effects of several n-alkanes on phospholipid hexagonal phases. Biophys. J. 74:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellens, H., D. P. Siegel, D. Alford, P. L. Yeagle, L. Boni, L. J. Lis, P. J. Quinn, and J. Bentz. 1989. Membrane fusion and inverted phases. Biochemistry. 28:3692–3703. [DOI] [PubMed] [Google Scholar]
- 50.Tilcock, C. P. S., P. R. Cullis, and S. M. Gruner. 1986. On the validity of P-31-NMR determinations of polymorphic phase behavior. Chem. Phys. Lipids. 40:47–56. [Google Scholar]
- 51.Gagné, J., L. Stamatatos, T. S. Diacovo, W. Hui, P. L. Yeagle, and J. R. Silvius. 1985. Physical properties and surface interactions of bilayer membranes containing N-methylated phosphatidylethanolamines. Biochemistry. 24:4400–4408. [DOI] [PubMed] [Google Scholar]
- 52.Siegel, D. P., J. Banschbach, D. Alford, H. Ellens, L. J. Lis, P. J. Quinn, P. L. Yeagle, and J. Bentz. 1989. b. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 28:3703–3709. [DOI] [PubMed] [Google Scholar]
- 53.Yeagle, P. L., R. M. Epand, C. D. Richardson, and T. D. Flanagan. 1991. Effects of the “fusion peptide” from measles virus on the structure of N-methyl dioleoylphosphatidylethanolamine membranes and their fusion with Sendai virus. Biochim. Biophys. Acta. 1065:49–53. [DOI] [PubMed] [Google Scholar]
- 54.van Gorkum, L. C. M., S. Q. Nie, and R. M. Epand. 1992. Hydrophobic lipid additives affect membrane stability and phase behavior of N-monomethyldioleoylphosphatidyl-ethanolamine. Biochemistry. 31:671–677. [DOI] [PubMed] [Google Scholar]
- 55.Epand, R. M., and R. F. Epand. 1994. Relationship between the infectivity of influenza virus and the ability of its fusion peptide to perturb bilayers. Biochem. Biophys. Res. Commun. 202:1420–1425. [DOI] [PubMed] [Google Scholar]
- 56.Davies, S. M. A., R. F. Epand, J. P. Bradshaw, and R. M. Epand. 1998. Modulation of lipid polymorphism by the feline leukemia virus fusion peptide: implications for the fusion mechanism. Biochemistry. 37:5720–5729. [DOI] [PubMed] [Google Scholar]
- 57.Tilcock, C. P. S., M. B. Bally, S. B. Farren, and P. R. Cullis. 1982. Influence of cholesterol on the structural preferences of dioleoylphosphatidylethanolamine dioleoylphosphatidylcholine systems: a P-31 and deuterium nuclear magnetic resonance study. Biochemistry. 21:4596–4601. [DOI] [PubMed] [Google Scholar]
- 58.Tenchov, B. G., R. C. MacDonald, and D. P. Siegel. 2006. Cubic phases in phosphatidylcholine-cholesterol mixtures: cholesterol as membrane “fusogen”. Biophys. J. 91:2508–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kozlov, M. M., and L. V. Chernomnordik. 1998. A mechanism of protein-mediated fusion: coupling between refolding of the influenza hemagglutinin and lipid rearrangements. Biophys. J. 75:1384–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, L., L. Ding, and H. W. Huang. 2003. New phases of phospholipids and implications to the membrane fusion problem. Biochemistry. 42:6631–6635. [DOI] [PubMed] [Google Scholar]
- 61.Efrat, A., L. V. Chernomordik, and M. M. Lozlov. 2007. Point-like protrusions as a prestalk intermediate in membrane fusion pathway. Biophys. J. 92:L61–L63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver, J. C., and R. A. Mintzer. 1981. Decreased bilayer stability due to transmembrane potentials. Phys. Lett. 86A:57–59. [Google Scholar]
- 63.Siegel, D. P., and R. M. Epand. 1997. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms. Biophys. J. 73:3088–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reference deleted in proof.
- 65.Tate, M. W., E. Shyamsunder, S. M. Gruner, and K. L. D'Amico. 1992. Kinetics of the lamellar-inverse hexagonal phase transition determined by time-resolved x-ray diffraction. Biochemistry. 31:1081–1092. [DOI] [PubMed] [Google Scholar]
- 66.Malinin, V. S., P. Frederik, and B. R. Lentz. 2002. Osmotic and curvature stress affect PEG-induced fusion of lipid vesicles but not mixing of their lipids. Biophys. J. 82:2090–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, Q., Y. Guo, L. Li, and S. W. Hui. 1997. Effects of lipid headgroup and packing stress on poly(ethylene glycol)-induced phospholipid vesicle aggregation and fusion. Biophys. J. 73:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kozlov, M. M., and M. Winterhalter. 1991. Elastic moduli and neutral surface for strongly curved monolayers. Analysis of experimental results. Journal de Physique II. 1:1085–1100. [Google Scholar]
- 69.Kozlov, M. M., S. Leikin, and R. P. Rand. 1994. Bending, hydration and interstitial energies quantitatively account for the hexagonal-lamellar-hexagonal reentrant phase transition in dioleoylphosphatidylethanolamine. Biophys. J. 67:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, J., A. Pekosz, and R. A. Lamb. 2000. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 74:4634–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blough, H. A. 1971. Fatty acid composition of individual phospholipids of influenza virus. J. Gen. Virol. 12:317–320. [DOI] [PubMed] [Google Scholar]
- 72.Rothman, J. E., D. K. Tsai, E. A. Dawidowicz, and J. Lenard. 1976. Transbilayer phospholipid asymmetry and its maintenance in the membrane of influenza virus. Biochemistry. 15:2361–2370. [DOI] [PubMed] [Google Scholar]
- 73.Kawasaki, K., and S. C. Ohnishi. 1992. Membrane fusion of influenza virus with phosphatidylcholine liposomes containing viral receptors. Biochem. Biophys. Res. Commun. 186:378–384. [DOI] [PubMed] [Google Scholar]
- 74.Stegmann, T. 1993. Influenza hemagluttinin-mediated membrane fusion does not involve inverted phase lipid intermediates. J. Biol. Chem. 268:1716–1722. [PubMed] [Google Scholar]
- 75.Alford, D., H. Ellens, and J. Bentz. 1994. Fusion of influenza virus with sialic acid-bearing target membranes. Biochemistry. 33:1977–1987. [DOI] [PubMed] [Google Scholar]
- 76.Shangguan, T., D. Alford, and J. Bentz. 1996. Influenza virus-liposome lipid mixing is leaky and largely insensitive to the material properties of the target membrane. Biochemistry. 35:4956–4965. [DOI] [PubMed] [Google Scholar]
- 77.Deutsch, J. W., and R. B. Kelly. 1981. Lipids of synaptic vesicles: relevance to the mechanism of membrane fusion. Biochemistry. 20:378–385. [DOI] [PubMed] [Google Scholar]
- 78.Takamori, S., M. Holt, K. Stenius, E. A. Lemke, M. Grønborg, D. Riedel, H. Urlaub, S. Schenk, B. Brügger, P. Ringler, S. A. Müller, B. Rammner, F. Gräter, J. S. Hub, B. L. de Groot, G. Mieskes, Y. Moriyama, J. Klingauf, H. Grubmüller, J. Heuser, F. Wieland, and R. Jahn. 2006. Molecular anatomy of a trafficking organelle. Cell. 127:831–846. [DOI] [PubMed] [Google Scholar]
- 79.Michaelson, D. M., G. Barkai, and Y. Barenholz. 1983. Asymmetry of lipid organization in cholinergic synaptic vesicle membranes. Biochem. J. 211:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wadel, K., E. Neher, and T. Sakaba. 2007. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron. 53:563–575. [DOI] [PubMed] [Google Scholar]
- 81.Wölfel, M., X. Lou, and R. Schneggenburger. 2007. A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse. J. Neurosci. 27:3198–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Churchward, M. A., T. Rogasevskaia, D. M. Brandman, H. Khosravani, P. Nava, J. K. Atkinson, and J. R. Coorssen. 2008. Specific lipids supply critical negative spontaneous curvature: an essential component of native Ca2+-triggered membrane fusion. Biophys. J. 94:3976–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Churchward, M. A., T. Rogasevskaia, J. Hofgen, J. Bau, and J. R. Coorssen. 2005. Cholesterol facilitates the native mechanism of Ca2+-trigered membrane fusion. J. Cell Sci. 118:4833–4848. [DOI] [PubMed] [Google Scholar]
- 84.Churchward, A., D. M. Brabdman, T. Rogasevskaia, and J. R. Coorssen. 2008. Copper (II) sulfate charring for high sensitivity on-plate fluorescent detection of lipids and sterols: quantitative analyses of the composition of functional secretory vesicles. J. Chem. Biol. DOI 10.1007/s12154-008-0007-1. [DOI] [PMC free article] [PubMed]
- 85.Zimmerberg, J., C. Sardet, and D. Epel. 1985. Exocytosis of sea urchin egg cortical vesicles in vitro is retarded by hyperosmotic sucrose: kinetics of fusion monitored by quantitative light-scattering microscopy. J. Cell Biol. 101:2398–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biswas, S., S.-R. Yin, P. S. Blank, and J. Zimmerberg. 2008. Cholesterol promotes hemifusion and pore widening in membrane fusion induced by influenza hemagglutinin. J. Gen. Physiol. 131:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farsad, K., and P. de Camilli. 2003. Mechanisms of membrane deformation. Curr. Opin. Cell Biol. 15:372–381. [DOI] [PubMed] [Google Scholar]
- 88.Zimmerberg, J., and M. M. Kozlov. 2006. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7:9–19. [DOI] [PubMed] [Google Scholar]
- 89.Martens, S., and H. T. McMahon. 2008. Mechanisms of membrane fusion: disparate players and common principles. Nat. Rev. Mol. Cell Biol. 9:543–556. [DOI] [PubMed] [Google Scholar]
- 90.Martens, S., M. M. Kozlov, and H. T. McMahon. 2007. How synaptotagmin promotes membrane fusion. Science. 316:1205–1208. [DOI] [PubMed] [Google Scholar]
- 91.Tristram-Nagle, S., and J. F. Nagle. 2007. HIV-1 fusion peptide decreases the bending energy and promotes curved fusion intermediates. Biophys. J. 93:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zemel, A., A. Ben-Shaul, and S. May. 2008. Modulation of the spontaneous curvature and bending rigidity of lipid membranes by interfacially adsorbed amphiphilic peptides. J. Phys. Chem. B. 112:6988–6996. [DOI] [PubMed] [Google Scholar]
- 93.Fournier, J. B. 1996. Nontopological saddle-splay and curvature instabilities from anisotropic membrane inclusions. Phys. Rev. Lett. 76:4436–4439. [DOI] [PubMed] [Google Scholar]
- 94.Kralj-Iglič, V., V. Heinrich, S. Svetina, and B. Žekš. 1999. Free energy of closed membrane with anisotropic inclusions. Eur. Phys. J. B. 10:5–8. [Google Scholar]
- 95.Chen, F. Y., M. T. Lee, and H. W. Huang. 2003. Evidence for membrane thinning effect as the mechanisms fro peptide-induced pore formation. Biophys. J. 84:3751–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Langosch, D., B. Brosig, and R. Pipkorn. 2001. Peptide mimetics of the vesicular stomatitis virus G-protein transmembrane segment drive membrane fusion in vitro. J. Biol. Chem. 276:32016–32021. [DOI] [PubMed] [Google Scholar]
- 97.Dennison, S. M., N. Greenfield, J. Lenard, and B. R. Lentz. 2002. VSV transmembrane domain (TMD) peptide promotes PEG-mediated fusion of liposomes in a conformationally sensitive fashion. Biochemistry. 41:14925–14934. [DOI] [PubMed] [Google Scholar]
- 98.Langosch, D., J. M. Crane, B. Brosig, A. Hellwig, L. K. Tamm, and J. Reed. 2001. Peptide mimetics of SNARE transmembrane segments drive membrane fusion depending on their conformational plasticity. J. Mol. Biol. 311:709–721. [DOI] [PubMed] [Google Scholar]
- 99.Hofman, M. W., K. Weise, J. Ollesch, P. Agrawal, H. Stalz, W. Stelzer, F. Hulsbergen, H. de Groot, K. Gerwert, J. Reed, and D. Langosch. 2004. De novo design of conformationally flexible transmembrane peptides driving membrane fusion. Proc. Natl. Acad. Sci. USA. 101:14776–14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siegel, D. P., V. Cherezov, D. V. Greathouse, R. E. Koeppe II, J. A. Killian, and M. C. Cafferty. 2006. Transmembrane peptides stabilize inverted cubic phases in a biphasic length-dependent manner: implications for protein-induced membrane fusion. Biophys. J. 90:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]