Abstract
The impact of a single seizure on cognition remains controversial. We hypothesized that a single early life seizure (sELS) on rat post-natal day (P) 7 would alter only hippocampal-dependent learning and memory in mature (P60) rats. The Morris Water Maze (MWM), Novel Object and Novel Place Recognition (NOR/NPR) tasks, and Contextual Fear Conditioning (CFC) were used to assess learning and memory associated with hippocampal/prefrontal cortex, perirhinal/hippocampal cortex, and amygdala function, respectively. The Elevated Plus Maze (EPM) and Open Field Test (OFT) were used to assess anxiety associated with the septum. We report that sELS impaired hippocampal-dependent short-term memory but not spatial learning or recall. sELS did not disrupt performance in the NOR/NPR. CFC performance suggested intact amydgala function. sELS did not change anxiety levels as measured by the EPM or OFT. Our data suggests that the long-term cognitive impacts of sELS are largely limited to the hippocampus/prefrontal cortex.
Keywords: seizure, morris water maze, development, kainate, working memory, hippocampus, pre-frontal cortex, neonate
Introduction
Nearly 3 in 1000 term infants and 15 in 1000 pre-term infants suffer from neonatal seizures (seizures occurring in the first month of life). It is estimated that 16% of these children develop learning disabilities mediated in part by the seizures themselves[1-4]. Despite therapeutic interventions[5], neonatal seizures are often repetitive and prolonged[6]. Severe neonatal seizures have been independently associated with an adverse developmental outcome[7]. Educating learning disabled children can cost 2 - 5 times that of their peers[8]. Less work has been done on understanding the impact of seizures on anxiety levels. Individuals suffering from epilepsy have a greater co-morbid incidence of anxiety disorders [9; 10] and attentional disorders[11]. Moderate neonatal encephalopathy, in which seizures are common, has been associated with attention deficit disorder and other disorders of executive function[12]. Despite the prevalence of seizure disorders in the neonatal population, the relationship between the seizures, the underlying pathologies and therapeutic side effects complicate our understanding of cognitive dysfunction following a single early life seizure (sELS).
Models of early life seizures can eliminate factors such as prior brain injury, medication effects, and behavioral interactions that impact learning ability. Multiple episodes (i.e., over several days) of early life seizures in developing rats result in later-life learning impairment that correlates with hippocampal cell loss and synaptic reorganization[13-15]. Comparatively, immature rats experiencing a single episode (i.e., over a single day) of early life seizures have not been found to suffer later-life behavioral alterations, cell loss, or synaptic reorganization[16-20]. Our recent work now demonstrates that a single episode of early life seizure following systemic injection of kainate impairs hippocampal-dependent episodic and/or working memory and synaptic plasticity through molecular alterations at excitatory synapses[21]. Alterations in hippocampal inhibitory synaptic transmission have also been linked to memory dysfunction in this model[22], however the molecular nature of this remains unclear. While early work has established that systemic kainate injections at this age activate primarily the hippocampus[23], behavioral testing helps to not only confirm these findings, but also determine if other structures are secondarily affected[17; 24].
It is well established that hippocampal dysfunction can disable certain forms of memory processes in behavioral testing[25]. Lesioning the rat hippocampus impairs long-term (hours to days) retention for spatial reference information[26], i.e. where an escape platform is located in reference to spatial visual cues. Hippocampal specific genetic alterations of glutamate receptors alter spatial learning[27]. Specific lesioning of the hippocampal CA3 region impairs spatial working memory due to this subregion’s critical role in pattern recognition[26]. Glutamate receptor subtypes distinguish the role of the hippocampus in mediating both short term (seconds to minutes) working memory and long-term spatial reference memory[28; 29]
The hippocampus also works closely with the pre-frontal cortex in the formation of short-term working memory[30; 31]. It now appears that the hippocampus and pre-frontal cortex may process spatial short-term information in parallel, compensating for each other when one is “off-line”[32] through discrete connections[33; 34]. Disruption of hippocampal-prefontal cortex circuits impairs spatial memory formation[35]. Thus, the hippocampus and pre-frontal cortex operate as a workspace for short-term information where the hippocampus may act as the input to the system.
Closely linked to hippocampal function is the perirhinal cortex[36]. Recognition memory, the preference for exploring a new object as opposed to a familiar object as studied with the Novel Object Recognition (NOR) test, selectively activates the perirhinal cortex[36] and is not impaired by hippocampal lesions[37]. Conversely, perirhinal lesions do not affect spatial recognition memory associated with novel places as tested with the Novel Place Recognition (NPR) test[38]. NPR performance relies on intact hippocampal function [36; 37; 39; 40].
Additional behavioral testing might indicate whether other extra-hippocampal structures are involved in rat models and/or indicate altered emotionality. Performance in the elevated plus maze (EPM) and open field test (OFT) depend primarily on the septum[41-44]. ELS induced by kainate or lithium-pilocarpine increase anxiety levels in rats when measured with the EPM[22; 45] but with variable results in the OFT[46]. Repetitive febrile seizures occurring early in life impair intermediate memory in the inhibitory avoidance task [47]. While these deficits were attributed to the hippocampus and septum, this task primarily involves the amygdala[48]. Increases in anxiety levels have also been noted in kindled adult rats[49]. Fear conditioning, a form of learning mediated primarily by the amygdala[50], has not been examined following ELS, however it is impaired following epileptogenesis in adult rats[51; 52].
Therefore, we tested the hypothesis that sELS occurring within the rat equivalent of the first month of human life (i.e. a neonatal seizure) would selectively impair hippocampal function but leave other behavioral functions intact in young adult (P60+) male rats. Kainate, a glutamate analog, was used to induce the single seizure because kainate injection results in a single discontinuous seizure with no evidence of subsequent recurrent seizures[21; 24; 53]. We have previously reported that sELS results in permanent changes in hippocampal-dependent short-term working and/or episodic memory[21]. In this paper, reanalysis of prior data confirms findings from the Morris Water Maze that these deficits are limited to short-term memory served by the hippocampus. We did not observe changes in other behavior tasks following sELS that could be attributed to structures beyond the hippocampus. Thus, sELS induced behavioral changes with mechanistic dysfunction isolated to the hippocampus and/or pre-frontal cortex.
Materials and Methods
Animals
All studies conformed to the requirements of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use subcommittees of the Denver VA Medical Center and the University of Colorado Health Sciences Center. Timed-pregnant Sprague Dawley rats (Charles Rivers Labs, Wilmington, MA) gave birth in-house. All rats were housed in micro-isolator cages with water and chow available ad libitum.
Seizure Induction
Kainate, a fixed glutamate analog[54], was used to induce temporal-lobe seizures[23]. Kainate injection leads to excess glutamate and thus produces a model of any insult that results in excess glutamate, including hypoxia[55]. Male rat pups were subcutaneously injected with kainate (1-2 mg/kg; Tocris, Ellisville, MO) on P7 (P0 defined as the date of birth) resulting in discontinuous behavioral and electrical seizure activity lasting for up to three hours. P7 is the most sensitive time in development for producing long-term effects from a sELS[22], and seizures induced at P7 are similar to human neonatal seizures clinically[6], biochemically[56], and electrographically[53]. Onset of seizure activity occurred within 30 min of injection and was characterized by intermittent myoclonic jerks, generalized tonic-clonic jerks, scratching, “swimming”, and “wet-dog shakes.” Mortality was less than 3%. Ictal bursts were typically less than 10 min and separated by 5-10 min[53] and not consistent with prior definitions of status epilepticus (ictal bursts > 20 min) in this model, which is associated with 8 times greater mortality[19]. Due to discrepancies in the definition of status epilepticus, which have included ictal discharges as brief as 5 min in adult humans[57], for clarity we do not use the term status epilepticus. Control male rat pups were injected with an equivalent volume of 0.9% saline. Male pups were chosen in order to eliminate the effects of hormonal cycles on behavior. Rats were then tagged using a commercially available microchip tagging system (Avid Identification Systems, Norco, CA) so that experimenters remained blinded to the treatment. Offspring were returned to their dam after observable seizure activity ceased. The dams were not observed to treat the seized pups differently than controls. Rats were weaned and separated according to gender at P22. At P60-90 behavioral analyses were undertaken with male rats. Four litters (average 10 pups/litter, 50% male) were used. Animals were consistently exposed to behavioral testing in the following order: Morris Water Maze, OFT, NOR/NPR, EPM, and CFC. This avoided generalization of fear response to other sensitive measures of hippocampal dependent behavior.
Morris Water Maze
Rats were trained in the standard hippocampal-dependent MWM (1.5-m diameter tank, 10-cm diameter platform submerged 1 cm below the surface of 21°C water obscured with black tempura paint (Prang, Heathrow, FL) as described previously[58]). Two blocks of 4 trials each occurred over 5 d. During each training trial, the amount of time spent in each quadrant, distance traveled, and latency to target platform were measured. If the rat did not find the target platform within 180 s, it was guided there and remained on the platform for 30 s. During the probe trials, the amount of time spent in each quadrant, distance traveled, latency to target platform, time spent in the target zone (a smaller region approximately 13 cm in diameter around the target location), and number of platform crossings were recorded. Automated data collection using WaterMaze (Actimetrics, Evanston, IL) incorporated a video camera connected to a personal computer.
Novel object and novel place recognition
The protocol for testing novel object and novel place recognition task has been described previously[40]. Briefly, a field 102 cm by 102 cm was constructed with 4 locations to mount objects approximately 20 cm from each corner. The walls were 20-cm high. A camera and software program from Actimetrics (LimeLight, Actimetrics Software, Evanston, IL) was used to analyze exploratory behavior. Each rat was habituated to the open field without objects present for 10 min on day 1. These data were used as an open field test (see below) to assess levels of anxiety (see below). Days 2 through 7 consisted of one trial per rat per day. Each trial consisted of a 5-min familiarization phase, followed by a 3-min intra-trial interval, and a 5-min test phase. For each rat, NOR and NPR tasks were interleaved on days 2 through 7. Thus, each rat was subjected to a total of three NOR trials and three NPR trials. For a NOR trial, the habituated rat was placed in the field with two non-odor retaining objects for 5 min during the familiarization phase. The objects used were a variety of common non-porous vinyl toys mounted on plastic stands to raise the object approximately 6 cm from the surface of the field. The rats were placed in a holding cage for 3 min between trials. When they were returned to the open field for the 5-min testing phase of the trial, a new non-porous object replaced one of the familiar objects. The novel object was semi-randomly selected such that every rat saw different objects during each NOR trial. For a NPR trial, the habituated rat was placed in the field with two non-odor retaining objects for 5 min during the familiarization phase. The rats were next placed in a holding cage for 3 min. When the rats were returned to the open field for the 5-min testing phase of the trial, one of the familiar objects was moved to a novel location in the open field. The novel location was randomized across trials. The amount of time spent with each object and the number of visits made to each object were measured during the testing phase for the NOR and NPR tasks. Sufficient objects were used so that each rat randomly saw novel objects each day of the NPR task.
Open Field Test
Motion capture software LimeLight (Actimetrics Software, Evanston, IL) was paired with cameras to analyze the behavior. A black painted field was used (102 cm × 102 cm, 20 cm). Each rat was placed in the middle of the open field and allowed to explore the arena freely for 600 s. The time spent near the walls versus the middle of the field was measured as a means to assess anxiety. The number of computer-generated grids crossed was measured as a means to assess basal motility. The field was cleaned thoroughly between rats using 1% Odor Mute (Heuter Toledo, Belleview, OH).
Elevated Plus Maze
Motion capture software LimeLight (Actimetrics Software, Evanston, IL) was paired with cameras to analyze the behavior. The EPM consisted of 4 arms situated at 90° angles to each other. Each arm was 114-cm long × 14 cm wide, two of the arms (situated across from each other) had 30-cm high walls (closed arms). The other two arms had a 1-cm high lip around the edge (open arms). At the start of the test, the rat was placed in the center of the arms. They were allowed to explore the maze for 5 min. The anxiety score was defined as time spent in the open arms/total time in the maze. The arms were cleaned thoroughly between rats using 1% Odor Mute.
Contextual Fear Conditioning
Rats were placed in a fear-conditioning chamber (30.5 cm × 24.1 cm × 21 cm, Med Associates, St. Albans, VT) with a grid floor (4.8-mm diameter rods, spaced 1.6 cm apart) connected to a constant current shocker (Med Associates, St. Albans, VT). Prior to placing each rat in the box, the box was sprayed with 3% acetic acid, which functioned as a specific odorant for the original context. Baseline freezing was measured for the initial 60 seconds. Two consecutive training blocks were administered. Each training block was 180 s long with a 30 s, 85 dB white noise conditioned stimulus (CS) and a 2 s, 0.5 mA footshock - unconditioned stimulus (US). The CS and US co-terminated at the end of the training block. All rats reacted to the footshock by jumping. The rats remained in the training box for 30 s following the second training block (“training”). The next day, the rats were returned to the training context for 6 min without white noise or footshock. The amount of freezing was measured for each rat (“context”). Three hours later, the rats were placed in a novel chamber (30.5 cm × 24.1 cm × 21 cm, smooth painted wood floor, red-tinted lighting, sprayed with 3% ammonium hydroxide) for 6 min (“altered context”), initially silent for 3 minutes (“no tone”) and then the CS was administered for the last 3 min (“tone”). Freezing was quantified automatically using a video-based conditioned fear testing system, FreezeFrame (Actimetrics Software, Evanston, IL). Freezing was defined as the lack of any movement except that required for respiration. The software package allowed for simultaneous visualization of the rats’ behavior and adjustment of a “freezing threshold” that defined the behavior as freezing or not freezing. The freezing threshold was confirmed separately for each rat, but tended to be consistent within a given apparatus. The experimenter assessing the freezing behavior was blind to the treatment groups. Each trial was divided into 1-s bins. Freezing was defined as no movement above the freezing threshold for the entire duration of the bin. Total freezing was summed over the length of the trial. Freezing is presented as the percent time spent freezing (time spent freezing/total time x 100).
Statistics
Data are expressed as mean ± SEM. Two-way repeated measures Analysis of Variance (ANOVAs) with Holm-Sidak post-hoc analysis was used, as indicated, for statistical comparisons for behavioral data using Origin 7.5 S5R (Origin Lab Corporation, Northhampton, MA) or SigmaStat (Systat, Point Richmond, CA). Significance is reported at P < 0.05 and N = number of rats.
Results
A sELS leaves spatial learning and recall intact but impairs shorter-term memory
Previous work has described the utility of the MWM in assessing hippocampal-dependent spatial reference learning and memory[58-60]. Therefore, to compare our results to other studies, we used the MWM to assess potential deficits induced by sELS on P7. As we have reported, rats experiencing sELS on P7 showed no significant differences across blocks of trials between sELS and controls[21], confirming normal spatial reference learning. Additionally, both groups of rats spent the majority of their time in the target quadrant during the training trials and the probe trails[21], confirming normal long-term spatial reference memory. These findings suggest that both control and sELS rats acquire and retain the MWM task equally. Furthermore, rats were assessed for swim speed using video-monitor tracking software and there were no significant differences between groups (data not shown).
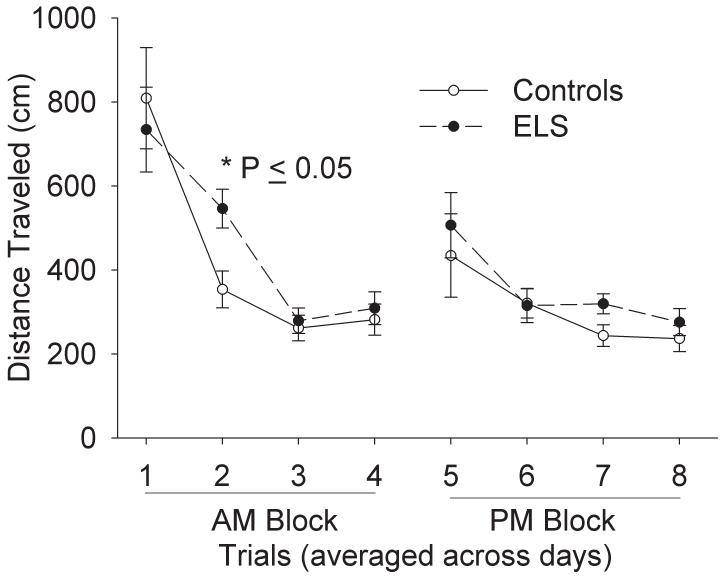
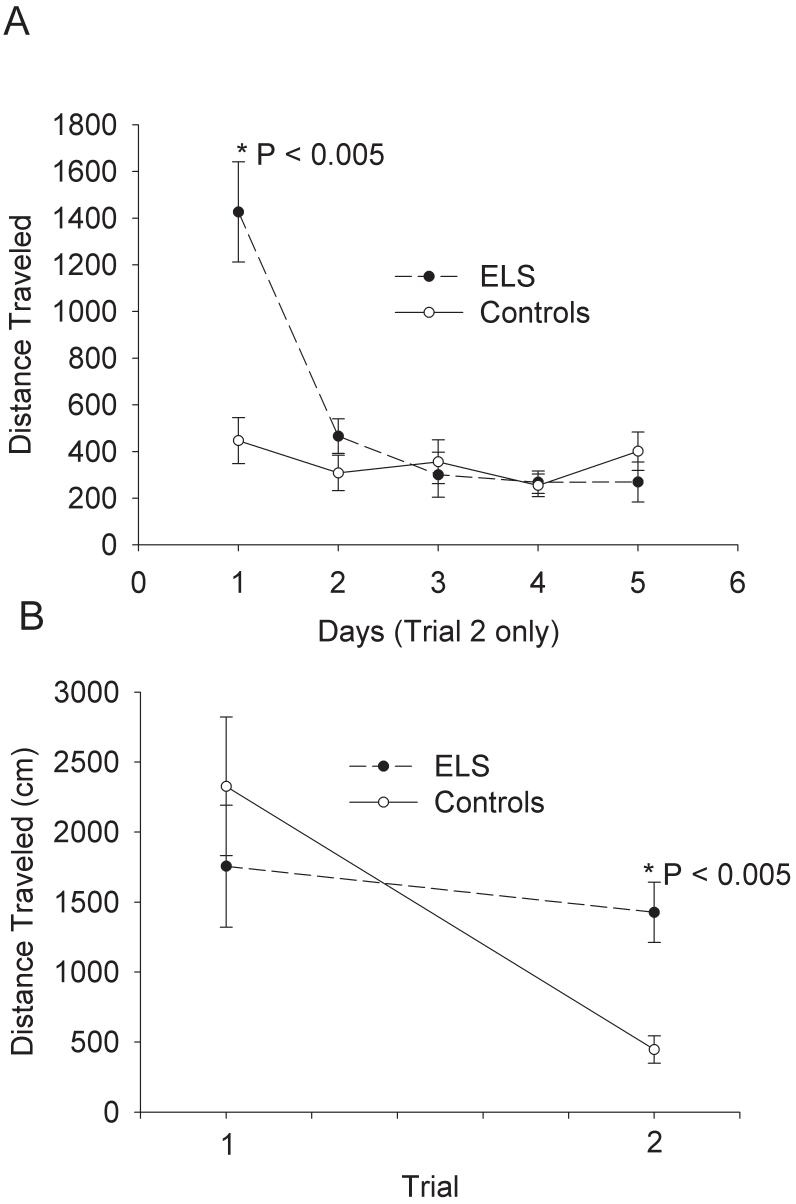
Further analysis of previously reported MWM data[21] confirmed an impairment of shorter-term memory following sELS. We examined each trial collapsed across all days of training. There was no overall difference as determined by two-way repeated measures ANOVA, but there was a statistically significant difference on trial 2 (Fig. 1). Control rats acquired the MWM task more rapidly by trial 2 than sELS rats; controls traveled less (353 ± 44 cm, N = 11) than sELS rats (546 ± 46 cm, N =10, two-way repeated measures ANOVA, P ≤ 0.05 by Holm-Sidak analysis). By examining only trial 2 across all days of training, the significant difference on trial 2 was due to errors occurring on day 1 of training (Fig. 2A). On trials 1 and 2 on day 1 of training, sELS rats (1427 ± 215 cm, N =10) traveled significantly farther on trial 2 compared to controls (447 ± 99 cm, N =11; two-way repeated measures ANOVA, P ≤ 0.05 by Holm-Sidak analysis; Fig. 2B). This behavioral deficit is consistent with a hippocampal-dependent malfunction of short-term spatial reference memory[21] that is observed with brief (30 s) inter-trial intervals [29].
Figure 1.
sELS impacts short-term memory in adult rats in the MWM. Following sELS, adult rats acquired the MWM task slower (546.1 ± 46.2 cm, N = 10) than saline injected controls (353.5 ± 43.4 cm, N = 11, P=0.04, two-way repeated ANOVA, Holm Sidak post-hoc) on trial two of 8 daily trials performed. Data are averaged across 5 days of training. From trial 3 onwards, no difference was seen compared to controls.
Figure 2.
sELS impacts short-term memory in adult rats in the MWM mostly on the first day of training. (A) Following sELS, adult rats traveled significantly further only on trial 2 of the first day of training (1426.8 ± 215.0 cm, N=10) compared to saline injected controls (446.2 ± 98.5 cm, N = 11, P < 0.005, two-way repeated ANOVA). No differences were found on subsequent days of training for trial 2. (B) On day 1, a comparison of trial 1 and trial 2 shows that the difference was present only on trial 2, showing that this is an isolated defect in short-term memory.
sELS does not alter object or place recognition memory
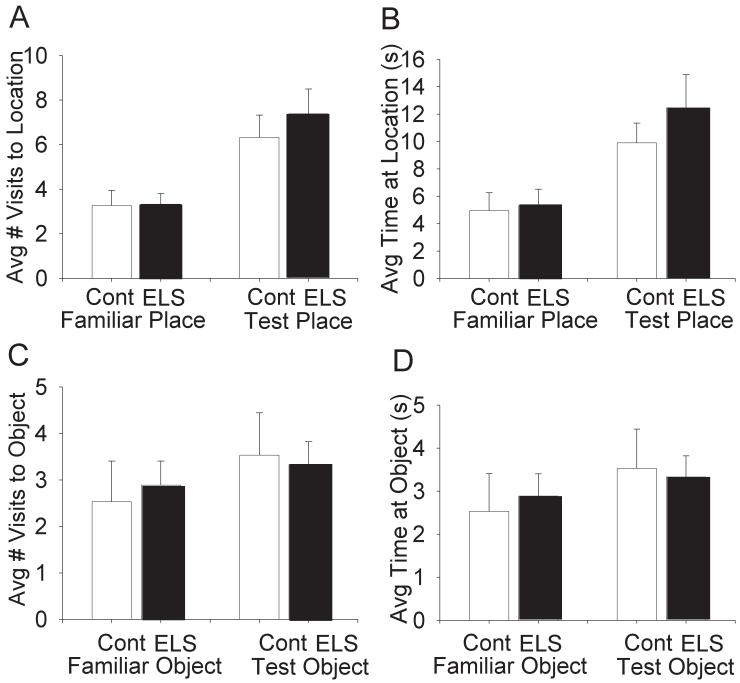
There were no significant differences between controls and sELS rats on NOR or NPR (Fig.3). The NPR task measures number of visits and time spent in either novel or familiar locations. It is expected that under normal conditions, novel locations will be visited more frequently and for longer periods of time than familiar locations. Both sELS (N = 18) and control (N = 15) rats differentially visited novel versus familiar locations with respect to number of visits (two-way repeated measures ANOVA, F(1,31) = 29.1, P < 0.001) and time (two-way repeated measures ANOVA, F(1,31) = 29.1, P < 0.001) but, there was no treatment effect (Fig. 3A,B).
Figure 3.
sELS did not impact NOR or NPR in adult rats. Following habituation in an open field, no differences were observed for exploration of a novel place (A,B) or novel object (C,D) in adult rats following sELS compared to saline-injected controls (Table 1).
The NOR task measures the number of visits and time spent near either novel or familiar objects. Under normal conditions, novel objects will be visited more frequently and for longer periods of time than familiar objects. Both sELS (N=6) and control (N=5) rats differentially visited novel versus familiar objects with respect to number of visits (two-way repeated measures ANOVA, F(1,9) = 5.3, P < 0.05) and time (two-way repeated measures ANOVA, F(1,9) = 12.1, P < 0.01) but, there was no treatment effect for either measure (Fig. 3C,D). These data suggest that an early life seizure does not negatively impact object or place recognition memory.
sELS does not impair formation and retention of amydala-dependent conditioned fear memory
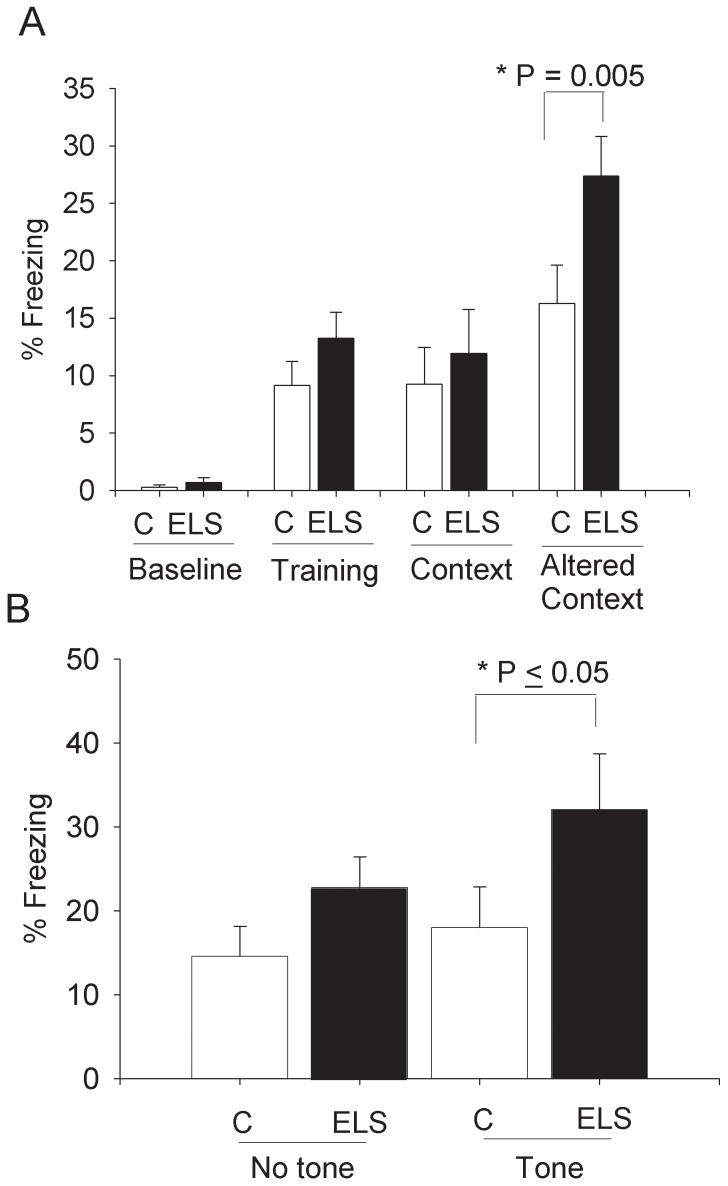
Baseline % freezing was not different between sELS (0.7 ± 0.4) and controls (0.3 ± 0.2; post-hoc Holm-Sidak, P=0.914). CFC training induced similar amounts of increased % freezing (“training”) between sELS (13.3 ± 2.3) and controls (9.2 ± 2.1; post-hoc Holm-Sidak, P=0.281). There were also similar amounts of % freezing following training with a context cue between sELS (11.9 ± 3.8) and control (9.2 ± 3.2; post-hoc Holm-Sidak, P=0.481) (Fig. 4A). This is consistent with normal amygdala function in the formation and retention of fear memory following sELS. There was, however, a significant interaction of treatment and altered context: sELS rats froze significantly more (27.4 % freezing ± 3.4) than controls (16.3 % freezing ± 3.3, post-hoc Holm-Sidak analysis, P ≤ 0.005) in the altered context (Fig. 4A). Examining the altered condition more specifically, we separated this condition into a “tone” versus “no tone” condition (Fig. 4B). There was an overall effect of sELS on freezing to the auditory cue (two-way ANOVA, F (1, 24) = 5.352, P = 0.030). The auditory tone drove the increased freezing in the altered context by sELS rats (32.0 % freezing ±6.7) versus controls rats (18.0 % freezing ± 4.9; post-hoc Holm-Sidak analysis, P ≤ 0.05). There was no difference for the “no tone” altered context (sELS rats 22.7 % freezing ± 3.7, versus controls rats 14.6 % freezing ± 3.6; post-hoc Holm-Sidak analysis, P = 0.242). sELS appears to have increased sensitivity to freezing elicited by an auditory cue. It is unlikely that this represents up-regulation of amygdala function but rather an alteration of other supporting structures, ie. hippocampus[61; 62] or other structures such as the septum or fimbria/fornix[44].
Figure 4.
Formation and retention of fear memory measured by CFC was not impaired in adult rats following sELS. Percent freezing indicates the relative time an animal freezes during the baseline condition, training condition, context condition, or an altered context condition. (A) Following training, all animals froze more than the baseline condition (post-hoc Holm-Sidak > 0.007). There were no significant differences following sELS (N = 13) or controls (N = 13) for percent time freezing at baseline, during training or in the context condition measuring retention. In the altered context, sELS rats froze significantly more (27.4 ± 3.4 % freezing, post-hoc Holm-Sidak analysis, p = 0.005) compared to controls (16.3 ± 3.3 % freezing). (B) Following sELS, in the altered context the auditory tone (“tone”) drives the increased freezing (32.0 ± 6.7, post-hoc Holm-Sidak, P = 0.047) compared to controls (18.0 ± 4.9 % freezing). No significant difference was found in the altered context in response to “no tone”.
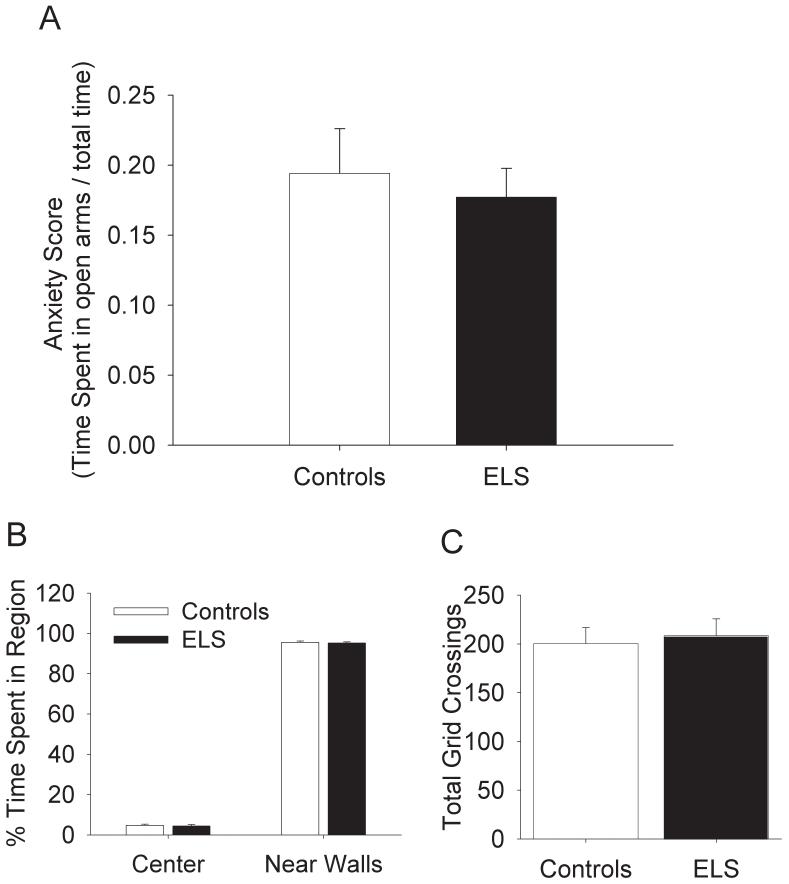
sELS does not alter septum-dependent anxiety as measured by the EPM and OFT
We used the EPM and OFT to ascertain if sELS rats experienced an altered degree of anxiety compared to control littermates. We did not see any significant differences between sELS rats (N = 25) and controls (N = 21) on either the EPM or the OFT (Fig. 5). In the EPM, both controls (Fig. 5A, 0.19 anxiety score ± 0.03) and sELS (0.18 anxiety score ± 0.038) rats had similar anxiety scores. In the OFT, both sELS and controls spent about the same amount of time in the center of the field (Fig. 5B, controls: 4.8% time in region ± 0.6 vs. sELS: 4.4% time in region ± 0.7) and near the walls (controls: 95.6% time in region ± 0.7 vs. sELS: 95.3% time in region ± 0.6). Also both sELS (Fig. 5C, 208.4 crossings ± 17.3) and controls (200.6 crossings ± 16.0) were equivocal on the number of grid crossings, suggesting that both rats were equally mobile.
Figure 5.
Anxiety was not altered in the EPM or OFT in adult rats following sELS. (A) No significant differences were detected in the EPM in controls (N=21) versus sELS (N=25). Greater percent time in open arms would reflect reduced anxiety. No significant differences were detected in the OFT in controls (N=21) versus sELS (N=25) for either percent time near walls (B) or total number of grid crossings (C).
Discussion
The effects of a single episode of early-life seizures in rodent models are inter-related to the severity of the seizure, developmental age, and technique. Following a relatively mild seizure in early life, rodents may have no cell loss but measurable behavioral abnormalities (reviewed by[24]). More severe and more prolonged seizures due to bicuculline at P4[63], direct intrahippocampal injection of kainate at P7[64], intraperitoneal injection of lithium pilocarpine at P12[45] and up[65], prolonged electrical stimulation after P14[66], or kainate injection at older ages[24] are each associated with overt cellular damage as well as behavioral abnormalities (reviewed by[24]). Like others [22; 46] we found that a relatively mild seizure induced by kainate in early life causes later-life behavioral deficits. The behavioral deficits described here and elsewhere[21], however, appear to be limited to short-term memory mediated by the hippocampus and possibly prefrontal cortex.
We report that sELS leaves long-term spatial reference memory intact, but causes a specific deficit in spatial short-term reference memory. Following sELS, mature rats are impaired in the early phases of learning a water maze task. The driving force behind the difference on trial two of the MWM (Fig.1) was the increased distance traveled by sELS rats on day 1 (Fig.2A). Looking only at day 1 data, the significant difference was largely due to the greater distance traveled on trial 2 (Fig. 2B). In other words, the ability to recall the location of the hidden platform over a short time-period was impaired by sELS. These experiments suggested that a single seizure could impair short-term memory function. Manipulation of information in short-term memory is often referred to as working memory[67]. Recent studies suggest that the hippocampus may underlie the “automatic recording” of memory [29] and be directly involved in working memory[28; 38]. To better resolve this issue, we have confirmed that working memory is impaired in rats experiencing sELS in the single-trial Radial Arm Water Maze [21]. This was observed by repeated errors while performing this task, i.e. the rats repeatedly entered arms already explored and found to lack the escape platform. Taken together with the data reported here, it is not surprising that the sELS rats have difficulty with spatial short-term memory. Specifically, recall of target locations on the first day of training in the MWM is a time frame where shorter-term memory is critical for learning.
Episodic memory encodes the when-where-what of past events[68]. Though it is debatable[69], single-trial memory may be a subset of episodic memory[29]. As we have shown[21], the specific deficit in spatial working memory in a single-trial task reflects hippocampal excitatory synaptic and receptor dysfunction and is consistent with knock-out [70-73] and pharmacological [74] studies. Further, dysfunction in the hippocampus could interfere with information flowing to the prefrontal cortex where it is manipulated and “held” to guide behavior [38]; the end result could be an impairment of working memory or short-term memory not unlike those observed here. Thus, the pre-frontal cortex, long thought to be a locus of working memory, may also have independent pathology as a result of sELS.
In this study we also investigated additional related hippocampal and perirhinal cortex functions: place and object recognition memory, the ability to respond to novel objects in an environment. We used the NOR and NPR task to ascertain if there were any differences between controls and sELS rats. There were no differences in the spatial learning task (NPR) or in recognition memory (NOR) (Fig. 3A-3D). While the information regarding maintenance of object recognition memory demonstrating an intact perirhinal cortex has not been presented before, the lack of impaired spatial learning and recall reflects and recapitulates our Radial Arm Water Maze data[21]. Thus, the NPR task appears to have confirmed our findings that sELS does not impair spatial learning or recall.
We did not find impaired formation of amygdala-dependent fear memory as assessed by standard CFC testing. Due to normal septal function, the abnormalities in fear memory that we did find cannot be explained by septal dysfunction. Rather, these enhanced fear memories (and possible altered emotionality) could be explained by hippocampal dysfunction, since the hippocampus is associated with the extinction of fear memory. This could be assessed in future studies addressing extinction by repeated testing in the altered context[61; 62].
We did not find altered anxiety associated with septal dysfunction in contrast to what has been reported by others[46]. Increasing anxiety has been observed interictally in kindled rats[49]. In dramatic contrast, recurrent seizures appeared to trigger a reduction in anxiety level as measured by the EPM[75]. Furthermore, there were no differences in the total number of grid crossings measured in the OFT (Fig. 5C). This data implies that sELS does not enhance anxiety levels nor is it anxiolytic.
The order that the rats completed the tasks could account for the differences in performance in the OFT or the EPM compared to prior results[46]. While we performed the CFC test last to avoid confounding other anxiety or memory measures, the act of subjecting these rats to multiple tests could influence performance in OFT and EPM.
In summary, the working memory deficit detected here is mostly due to hippocampal dysfunction. We speculate that the mechanisms involved in hippocampal synaptic plasticity may also be somehow altered to produce the behavioral phenotype observed here. To that end, a loss of LTP [21; 22] and enhancement of LTD[21] following sELS have been described. These changes in hippocampal physiology are also associated with changes in hippocampal dependent behavior[21; 22; 46]. Thus, because the importance of the hippocampus in working memory has been established, any sELS-induced long-term changes that occur at the hippocampal synapses could help explain the altered memory. Although these initial reports regarding behavioral changes after sELS are informative, future work may be necessary to further characterize the behavioral impact of sELS. Clinically, specific neuropsychologic measurements of working memory may be necessary to define and treat pediatric patients following sELS.
Acknowledgements
We would like to acknowledge the following for their contributions: Dr. Richard Radcliffe, Elizabeth Stubblefield, and Chrissy Algeier. This work was supported by the NIMH/APA Diversity Program in Neuroscience (MH18882-17), the VA Medical Research Service (MHM), and the NIH/NINDS (NS041267, NS056090 (TAB), AG00961 (MHM)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dixon G, Badawi N, Kurinczuk JJ, Keogh JM, Silburn SR, Zubrick SR, et al. Early developmental outcomes after newborn encephalopathy. Pediatrics. 2002;109(1):26–33. doi: 10.1542/peds.109.1.26. [DOI] [PubMed] [Google Scholar]
- [2].Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58(4):542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- [3].Rennie JM, Boylan GB. Neonatal seizures and their treatment. Curr Opin Neurol. 2003;16:177–181. doi: 10.1097/01.wco.0000063768.15877.23. [DOI] [PubMed] [Google Scholar]
- [4].Pisani F, Cerminara C, Fusco C, Sisti L. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology. 2007;69(23):2177–2185. doi: 10.1212/01.wnl.0000295674.34193.9e. [DOI] [PubMed] [Google Scholar]
- [5].Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. New Engl J Med. 1999;341(7):485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- [6].Arzimanoglou A, Guerrini R, Aicardi J. Neonatal seizures. Aicardi’s Epilepsy in Children. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 191–192. [Google Scholar]
- [7].Ortibus EL, Sum JM, Hahn JS. Predictive value of EEG for outcome and epilepsy following neonatal seizures. Electroencephalogr Clin Neurophysiol. 1996;99:175–185. doi: 10.1016/0013-4694(95)00245-6. [DOI] [PubMed] [Google Scholar]
- [8].Burke M. Education commision of the States, report on Special Education-Finance. 2003 www.ecs.org.
- [9].Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54(4):425–432. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- [10].Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol. 2003;45:292–295. doi: 10.1017/s0012162203000550. [DOI] [PubMed] [Google Scholar]
- [11].Dunn DW, Austin JK, Harezlak J, Ambrosius WT. ADHD and epilepsy in childhood. Dev Med Child Neurol. 2003;45:50–54. [PubMed] [Google Scholar]
- [12].Linstrom K, Lagerroos P, Gillberg C, Fernell E. Teenage outcome after being born at term with moderat neonatal encephalopathy. Pediatr Neurol. 2006;35:268–274. doi: 10.1016/j.pediatrneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- [13].Sarkisian MR, Tandon P, Liu Z, Yang Y, Hori A, Holmes GL, et al. Multiple kainic acid seizures in the immature and adult brain: ictal manifestations and long-term effects on learning and memory. Epilepsia. 1997;38:1157–1166. doi: 10.1111/j.1528-1157.1997.tb01211.x. [DOI] [PubMed] [Google Scholar]
- [14].Chang YC, Kuo YM, Huang AM, Huang CC. Repetitive febrile seizures in rat pups cause long-lasting deficits in synaptic plasticity and NR2A tyrosine phosphorylation. Neurobiol Disease. 2005;18:466–475. doi: 10.1016/j.nbd.2004.12.012. [DOI] [PubMed] [Google Scholar]
- [15].Swann JW. The effects of seizures on the connectivity and circuitry of the developing brain. Ment Retard Dev Disabil Res Rev. 2004;10:96–100. doi: 10.1002/mrdd.20018. [DOI] [PubMed] [Google Scholar]
- [16].Nitecka L, Tremblay E, Charton G, Bouillot JP, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage in the rat. II. Histopathological sequelae. Neuroscience. 1984;13:1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- [17].Stafstrom CE, Chronopoulos A, Thurber S, Thompson JL, Holmes GL. Age-dependent cognitive and behavioral deficits after kainic acid seizures. Epilepsia. 1993;34(3):420–432. doi: 10.1111/j.1528-1157.1993.tb02582.x. [DOI] [PubMed] [Google Scholar]
- [18].Holmes GL, Gairsa J-L, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- [19].Stafstrom CE, Thompson JL, Holmes GL. Kaininc acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Brain Res Dev Brain Res. 1992;21:227–236. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- [20].Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshe SL. Resistance of immature hippocampus to morphological and physiological alterations following status epilepticus and kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- [21].Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol. 2007;61(5):411–426. doi: 10.1002/ana.21071. [DOI] [PubMed] [Google Scholar]
- [22].Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci. 2000;12:2252–2264. doi: 10.1046/j.1460-9568.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- [23].Tremblay E, Nitecka L, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. I. Clinical, electrographic and metabolic observations. Neuroscience. 1984;13:1051–1072. doi: 10.1016/0306-4522(84)90288-4. [DOI] [PubMed] [Google Scholar]
- [24].Stafstrom CE. Assessing the behavioral and cognitive effects of seizures on the developing brain. Prog Brain Res. 2002;135:377–390. doi: 10.1016/S0079-6123(02)35034-9. [DOI] [PubMed] [Google Scholar]
- [25].Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- [26].Kesner RP, Farnsworth G, Kametani H. Role of parietal cortex and hippocampus in representing spatial information. Cereb Cortex. 1991;1(5):367–373. doi: 10.1093/cercor/1.5.367. [DOI] [PubMed] [Google Scholar]
- [27].Tsien JZ. Linking Hebb’s coincidence-detection to memory formation. Curr Opin Neurobiol. 2000;10:266–273. doi: 10.1016/s0959-4388(00)00070-2. [DOI] [PubMed] [Google Scholar]
- [28].Bast T, daSilva BM, Morris RGM. Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci. 2005;25:5845–5856. doi: 10.1523/JNEUROSCI.0698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morris RGM, Frey U. Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience. Philos Trans R Soc Lond B Biol Sci. 1997;352(1360):1489–1503. doi: 10.1098/rstb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aggleton JP, Hunt PR, Rawlins JNP. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- [31].Becker JT, Morris RG. Working memory(s) Brain Cogn. 1999;41:1–8. doi: 10.1006/brcg.1998.1092. [DOI] [PubMed] [Google Scholar]
- [32].Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res. 2006;169(1):142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- [33].Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci. 2003;23(4):1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Laroche S, Jay TM, Thierry A-M. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci Lett. 1990;114:184–190. doi: 10.1016/0304-3940(90)90069-l. [DOI] [PubMed] [Google Scholar]
- [35].Floresco SB, Seamans JK, Phillips AG. Selective roles of hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005;58B:218–233. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- [37].Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- [38].Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10(4):438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [39].Aggleton JP, Kyd RJ, Bilkey DK. When is the perirhinal cortex necessary for the performance of spatial memory tasks? Neurosci Biobehav R. 2004;28(6):611–624. doi: 10.1016/j.neubiorev.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [40].Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cheeta S, Kenny PJ, File SE. Hippocampal and septal injections of nicotine and 8-OH-DPAT distinguish among different animal tests of anxiety. Prog Neuropsychpharmacol Biol Psychiatry. 2000;24:1053–1067. doi: 10.1016/s0278-5846(00)00129-9. [DOI] [PubMed] [Google Scholar]
- [42].Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci Biobehav Reviews. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- [43].Brito GN, Brito LS. Septohippocampal system and the prelimbic sector of frontal cortex: a neuropsychological battery analysis in the rat. Behav Brain Res. 1990;36:127–146. doi: 10.1016/0166-4328(90)90167-d. [DOI] [PubMed] [Google Scholar]
- [44].Decker MW, Curzon P, Brioni JD. Influence of separate and combined septal and amygdala lesions on memory, acoustic startle, anxiety and locomotor activity in rats. Neurobiol Learn Mem. 1995;64:156–168. doi: 10.1006/nlme.1995.1055. [DOI] [PubMed] [Google Scholar]
- [45].Kubova H, Mares P, Suchomelova L, Brozek G, Druga R, Pitkanen A. Status epilepticus in immature rats leads to behavioral and cognitive impairment and epileptogenesis. Eur J Neurosci. 2004;19:3255–3265. doi: 10.1111/j.0953-816X.2004.03410.x. [DOI] [PubMed] [Google Scholar]
- [46].Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45(12):1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- [47].Chang Y-C, Huang A-M, Kuo Y-M, Wang S-T, Chang Y-Y, Huang C-C. Febrile seizures impair memory and cAMP response-element binding protein activation. Ann Neurol. 2003;54(6):706–718. doi: 10.1002/ana.10789. [DOI] [PubMed] [Google Scholar]
- [48].McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: Interaction with other brain systems. Proc Natl Acad Sci USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kalynchuk LE, Pinel JP, Treit D, Barnes SJ, McEachern JC, Kippin TE. Persistence of the interictal emotionality produced by long-term amygdala kindling in rats. Neuroscience. 1998;85(4):1311–1319. doi: 10.1016/s0306-4522(98)00003-7. [DOI] [PubMed] [Google Scholar]
- [50].Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- [51].Kemppainen EJS, Nissinen J, Pitkänen A. Fear conditioning is impaired in systemic kainic acid and amygdala-stimulation models of epilepsy. Epilepsia. 2006;47:820–829. doi: 10.1111/j.1528-1167.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- [52].Szyndler J, Wierzba-Bobrowicz T, Skorzewska A, Maciejak P, Walkowiak J, Lechowicz W, et al. Behavioral, biochemical and histological studies in a model of pilocarpine-induced spontaneous recurrent seizures. Pharmacol Biochem Behav. 2005;81(1):15–23. doi: 10.1016/j.pbb.2005.01.020. [DOI] [PubMed] [Google Scholar]
- [53].Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11(11):1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- [54].Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channel. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- [55].Yager JY, Armstrong EA, Miyashita H, Wirrell EC. Prolonged neonatal seizures exacerbate hypoxic-ischemic brain damage: correlation wiht cerebral energy metabolism and excitatory amino acid release. Dev Neurosci. 2002;24:367–381. doi: 10.1159/000069049. [DOI] [PubMed] [Google Scholar]
- [56].Talos DM, Follet PL, Folkerth RD, Fishman RE, Trachtenberg FL, Volpe JJ, et al. Developmental regulation of AMPA receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury: Part II. Human cerebral white matter and cortex. J Comp Neurol. 2006;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lowenstein DH. Status epilepticus: an overview of the clinical problem. Epilepsia. 1999;40(Suppl 1):S3–S8. doi: 10.1111/j.1528-1157.1999.tb00872.x. [DOI] [PubMed] [Google Scholar]
- [58].Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- [59].Eichenbaum H, Stewart C, Morris RGM. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- [61].Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [62].Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- [63].Wasterlain C. Effects of neonatal status epilepticus on rat brain development. Neurology. 1976;26:975–986. doi: 10.1212/wnl.26.10.975. [DOI] [PubMed] [Google Scholar]
- [64].Leite JP, Babb TL, Pretorius JK, Kuhlman PA, Yeoman KM, Mathern GW. Neuron loss, mossy fiber sprouting, and interictal spikes after intrahippocampal kainate in developing rats. Epilepsy Res. 1996;26:219–231. doi: 10.1016/s0920-1211(96)00055-1. [DOI] [PubMed] [Google Scholar]
- [65].Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Thompson K, Holm AM, Schousboe A, Popper P, Micevych P, Wasterlain C. Hippocampal stimulation produces neuronal death in the immature brain. Neuroscience. 1998;82:337–348. doi: 10.1016/s0306-4522(97)00195-4. [DOI] [PubMed] [Google Scholar]
- [67].Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- [68].Morris RG. Episodic-like memory in animals: psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci. 2001;356:1453–1465. doi: 10.1098/rstb.2001.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Roberts WA, Feeney MC, MacPherson K, Petter M, McMillan N, Musolino E. Episodic-like memory in rats: is it based on when or how long ago? Science. 2008;320:113–115. doi: 10.1126/science.1152709. [DOI] [PubMed] [Google Scholar]
- [70].Reisel D, Bannerman DM, Schmitt WB, Deacon RMJ, Flint J, Borchardt T, et al. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5(9):868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- [71].Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RMJ, et al. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat Neurosci. 2005;8(3):270–272. doi: 10.1038/nn1412. [DOI] [PubMed] [Google Scholar]
- [72].Reisel D, Bannerman DM, Deacon RMJ, Sprengel R, Seeburg PH. GluR-A dependent synaptic plasticity is required for the temporal coding of non-spatial information. Behav Neurosci. 2005;119:1298–1306. doi: 10.1037/0735-7044.119.5.1298. [DOI] [PubMed] [Google Scholar]
- [73].Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, et al. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci. 2008;28(14):3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lee HK, Takamiya K, Han J-S, Man H, Kim C-H, Rumbaugh G, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- [75].Detour J, Schroeder H, Desor D, Nehlig A. A 5-month period of epilepsy impairs spatial memory, decreases anxiety, but spares object recognition in the lithium-pilocarpine model in adult rats. Epilepsia. 2005;46(4):499–508. doi: 10.1111/j.0013-9580.2005.38704.x. [DOI] [PubMed] [Google Scholar]