Abstract
Prolonged treatment of leukemic cells with chemotherapeutic agents frequently results in development of drug resistance. Moreover, selection of drug-resistant cell populations may be associated with changes in malignant properties such as proliferation rate, invasiveness, and immunogenicity. In the present study, the sensitivity of cytarabine (1-β-d-arabinofuranosylcytosine, araC)-resistant and parental human leukemic cell lines (T-lymphoid H9 and acute T-lymphoblastic leukemia Molt-4) to natural killer (NK) cell-mediated killing was investigated. The results obtained demonstrate that araC-resistant H9 and Molt-4 (H9rARAC100 and Molt-4rARAC100) cell lines are more sensitive to NK cell-mediated lysis than their respective parental cell lines. This increased sensitivity was associated with a higher surface expression of ligands for the NK cell-activating receptor NKG2D, notably UL16 binding protein-2 (ULBP-2) and ULBP-3 in H9rARAC100 and Molt-4rARAC100 cell lines. Blocking ULBP-2 and ULBP-3 or NKG2D with monoclonal antibody completely abrogated NK cell lysis. Constitutive phosphorylated extracellular signal-regulated kinase (ERK) but not pAKT was higher in araC-resistant cells than in parental cell lines. Inhibition of ERK using ERK inhibitor PD98059 decreased both ULBP-2/ULBP-3 expression and NK cell cytotoxicity. Furthermore, overexpression of constitutively active ERK in H9 parental cells resulted in increased ULBP-2/ULBP-3 expression and enhanced NK cell lysis. These results demonstrate that increased sensitivity of araC-resistant leukemic cells to NK cell lysis is caused by higher NKG2D ligand expression, resulting from more active ERK signaling pathway.
Introduction
Nucleoside analogs represent a group of cytotoxic antimetabolites in the treatment of hematological malignancies, solid tumors and viral infections [1–4]. They mimic physiological nucleosides and share their metabolic pathways. Cytarabine (1-β-d-arabinofuranosylcytosine, araC), a deoxycytidine analog, is one of the most important anti-leukemic drugs currently available for the treatment of acute myeloid leukemia [5,6], relapsed and refractory acute lymphoblastic leukemia [7–9], and large cell lymphoma [10]. Prolonged, in vitro and in vivo, treatment with araC has, however, resulted in the emergence of drug resistant cells with diminished sensitivity to the drug and ultimately contributing to treatment failures [6,11–13].
Acquired drug resistance of leukemic cells caused by pretreatment with cytostatic drugs influences the sensitivity of leukemic cells toward cytotoxic lymphocytes [14–19]. Whereas some reports show decreased sensitivity of drug-resistant leukemic cells to cellular cytotoxicity [17,18,20,21], Posovszky et al. [19] reported that chemotherapeutic drugs including araC sensitize pre-B acute lymphoblastic leukemia (ALL) cells for CD95- and cytotoxic T-lymphocyte-mediated apoptosis. Recent studies have shown that natural killer (NK) cells display cytolytic activities by engagement of receptors involved in NK cell activation and inhibition [22]. Although NK cells can kill target cells spontaneously without prior stimulation, a delicate balance between signaling through inhibitory [killer immunoglobulin-like receptors (KIR), CD94-NK group 2, member A (NKG2A)] and activating receptors [natural cytotoxic receptors (NCRs-NKp30, NKp44, and NKp46), NK group 2, member D (NKG2D), and DNAX accessory molecule-1 (DNAM-1)] tightly regulates their activation [22].
The relevance of the NKG2D/NKG2D ligand system for the immune surveillance in patient leukemia cells was previously described [23]. Salih et al. [23] reported that leukemia cells from patients variously express major histocompatibility complex class I (MHC-I)-related chain A/B (MICA/B) and UL16 binding protein (ULBP). They also showed that patient leukemia cells were lysed by NK cells in an NKG2D-dependent fashion. The NKG2D receptor is constitutively expressed on the cell surface of human NK cells [24], γδT cells, and CD8+ αβT cells [25]. The proposed role of the NKG2D receptor in innate and adaptive immune responses to cellular and tissue stress is based on the ability of the receptor to stimulate cytotoxic effects of NK cells and T cells against virally infected cells and tumor cells in vitro and in vivo [26]. Specifically, NKG2D receptor activation can induce target cell lysis and trigger the production of cytokines [27,28] and chemokines [27,29,30], as well as perforin and granzyme involved in cellular lysis [31]. DNAX accessory molecule-1, a coactivating receptor of NK cells, is another surface molecule that has been shown to participate in NK cell activation [32,33]. DNAX accessory molecule-1 is known to be involved not only in NK cell activation but also in cell-cell adhesion [34]. It has been shown that the susceptibility of tumor cells to NK cell-mediated lysis is dependent on the expression level of polio virus receptor (PVR) specifically recognized by DNAM-1 [32,33,35,36].
In this study, the expression of ligands of NK cell-activating and -inhibitory receptors on parental and H9rARAC100 and Molt-4rARAC100 cell lines and their function in NK cell-mediated cytolytic activity were investigated. The possible mechanism involved in the expression pattern of the ligands was also studied.
Materials and Methods
Cell Lines
The human T cell lymphoma H9 and acute T lymphoblastic leukemia Molt-4 cell lines were obtained from the American Type Culture Collection (Rockville, MD; ATCC Nos. HTB-176 and CRL 1582, respectively). The araC-resistant H9 and Molt-4 cell sublines were established by exposing parental cells to increasing concentrations of the drug. The resistant sublines were grown for more than 6 months in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% fetal calf serum (FCS) and containing 100 µM araC (designated H9rARAC100 and Molt-4rARAC100, respectively). All experiments were performed using araC-resistant cells subcultured at 5-day intervals without further addition of drug. All culture media and media supplements were purchased from Seromed (Berlin, Germany). The cells were propagated in IMDM supplemented with 10% FCS, 100 IU/ml penicillin, and 100 mg/ml streptomycin at 37°C in a humidified 5% CO2 incubator. The NK cell sensitive erythroleukemic cell line K562 (ATCC No. CCL-243) was maintained in IMDM supplemented with 20% FCS and used as control for NK cell cytotoxicity.
MTT Assay
Cell viability was investigated using the modified 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium-bromide (MTT) assay as previously described [37]. Briefly, cells were grown in 96-well plates with and without addition of drugs. After the incubation period, MTT reagent was added for 4 hours. Thereafter, 100 µl of sodium dodecyl sulfate (SDS) solution (20% SDS in a 1:1 dimethyformamide/H2O solution) was added for a further 4 hours. Plates were read on a multi-well scanning spectrophotometer (Tecan, Crailsheim, Germany) at a wavelength of 560 nm and a reference wavelength of 620 nm. Cell viability was determined as the relative reduction of the amount of MTT reduced by cells to its purple formazan derivative, which correlates with the amount of viable cells in relation to cell control.
Polyclonal NK Cells Preparation
Human peripheral blood mononuclear cells were isolated from the blood of healthy volunteers by Ficoll-Hypaque centrifugation. Freshly isolated peripheral blood mononuclear cells were incubated for 2 hours at 37°C to allow adherence of monocytes to the bottom of the culture flasks. The cell suspension was collected and NK cells were separated according to manufacturer's protocol using the MACS NK cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany). The separated NK cells were treated with 100 U/ml recombinant human interleukin-2 (IL-2; Cell Concepts, Umkirch, Germany) for 5 days. Flow cytometric analysis to determine the purity of NK cells showed that more than 90% of the cells were CD56+CD3- (data not shown).
Antibodies, Reagents, and Measurement of Cell Surface Receptors
AraC was obtained from Sigma (Deisenhofen, Germany), the ERK inhibitor PD98059 was from Calbiochem (Darmstadt, Germany), and purified NKG2D was from BD Pharmingen (San Diego, CA). For quantitative analysis of the expression of PVR, MICA/B, ULBP-1, ULBP-2, ULBP-3, and MHC-I, a one-color cytofluorometric analysis (FACScan; Becton Dickinson, Heidelberg, Germany) was carried out. Cells were stained with mouse monoclonal to PVR, ab3142 (Abcam, Cambridge, UK), antihuman MICA/MICB monoclonal antibody (mAb), BAMO1 (Immatics, Tuebingen, Germany), ULBP1-3 (R&D Systems, Wiesbaden, Germany) followed by phycoerythrin (PE)-conjugated goat antimouse IgG second reagent (R&D Systems). Fluorescein isothiocyanate-conjugated mouse monoclonal antihuman HLA class I (Biosource, CA) was used for MHC-I expression measurements.
Natural Killer Cytotoxicity Assay
Natural killer cells were tested for cytolytic activity against indicated target cells using the “aCella-Tox” kit (Cell Technology, Mountain View, CA) as previously described [36]. Briefly, target cells were plated in triplicate (5000 cells per well) in a 96-well white plate (Greiner Bio-One, Frickenhausen, Germany). Effector cells (NK cells) at indicated effector to target (E/T) ratios were added. Spontaneous effector and target cell death was accomplished by including control wells of effector and target cells at numbers corresponding to those of their various E/T ratios. To determine maximum release [total glyceralehyde-3-phosphate dehydrogenase (G3PDH) released], 10 µl of lytic reagent (0.5% NP-40/100 µl sample) was added to the target cells positive control 10 minutes to end of assay incubation. At the end of 4 hours of incubation, 100 µl of 2x enzyme assay reagent was added to each well. Fifty microliters of 1x detection reagent was immediately added to each well. The plate was read at once in a luminometer (Glomax; Promega, Mannheim, Germany). For the mAb-mediated neutralization experiments, NK cells were first incubated for 1 hour with 50% human serum (to prevent binding of the mAbs to the various Fc receptors expressed on the surface of human NK cells) and washed. Leukemic and NK cells were then incubated initially at room temperature for 20 minutes with 20-µg/ml indicated mAb. End concentration of mAb for the 4-hour assay was 4 µg/ml. Isotype control IgG (Becton Dickinson, San Jose, CA) was used as negative control. Iscove's modified Dulbecco's medium supplemented with 1% heat-inactivated FCS was used as assay medium. The percent cytotoxicity was calculated as follows: [(experimental G3PDH release - spontaneous G3PDH release from effector cells alone - spontaneous G3PDH release from target cells alone) / (maximum G3PDH release from target cells - spontaneous G3PDH release from target cells)] x 100. The spontaneous target cell release was always <20% of maximum release.
Real-time Polymerase Chain Reaction
Total RNA was isolated using TRI reagent (Sigma-Aldrich, Seelze, Germany) according to the manufacturer's protocol. Reverse transcription was carried out with reagents from Applied Biosystems (Foster City, CA) according to the manufacturer's instructions. cDNA was quantified by quantitative real-time polymerase chain reaction (PCR) with the ABI PRISM 7900HT. All reactions were performed using SYBR Green assays according to a standard thermal profile: denaturation at 95°C for 15 seconds, annealing/extension at 60°C for 60 seconds with 40 repeats. Primers for ULBP-2 are as follows: forward 5′-CCC TGG GGA AGA AAC TAA ATG TC-3′; reverse 5′-ACT GAA CTG CCA AGATCC ACT GCT-3′. Primers for ULBP-3 are as follows: forward 5′-AGA TGC CTG GGG AAA ACA ACT G-3′; reverse 5′-GTA TCC ATC GGC TTC ACA CTC ACA-3′. Primers for β-actin are as follows: forward 5′-CGC GAG AAG ATG ACC CAG AT-3′; reverse 5′-CAG AGG CGT ACA GGG ATA GCA-3′. All the samples were performed at least in duplicates. Threshold levels and baseline were optimized. Relative quantification was determined with the SDS2.1 software (Applied Biosystems) provided with the ABI PRISM 7900HT (ΔΔCt method). Normalization was obtained by using β-actin as endogenous control and the parental cell culture as calibration sample in comparison to the araC-resistant cell cultures. The results are presented as fold increase.
Western Blot
Western blot analysis was performed as previously described [38]. Briefly, cell lysates were subjected to SDS-PAGE before transferring to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) using the Mini-Protean II System (Bio-Rad, Munich, Germany). After transfer, blots were blocked in Tris-buffered saline blocking buffer containing 3% bovine serum albumin for 1 hour at room temperature to saturate the nonspecific protein-binding sites on the nitrocellulose membrane. The following primary rabbit polyclonal Abs were used: ERK, anti-phospho-ERK1/2, AKT, anti-phospho-AKT (ser 473; Cell Signaling, Beverly, MA). Mouse polyclonal β-actin Ab was from Sigma-Aldrich (Munich, Germany). The blots were incubated overnight with the primary Ab diluted in Tris-buffered saline at 4°C with gentle agitation. After a 1-hour incubation period with peroxidase-conjugated secondary Ab at room temperature, visualization was performed by enhanced chemiluminescence using a commercially available kit (Amersham, Liverpool, UK).
Inhibition of ERK Activation
Inhibition of ERK activation was performed by pretreating both parental and araC-resistant cells for 48 hours with 20 µM PD98059.
Transfection and Plasmid
The constitutively active ERK 2 expression plasmid was kindly provided by Professor Stefanie Dimmeler (Frankfurt, Germany). Plasmids included (pcDNA3.1; empty vector) as a control and constitutively active ERK (pcDNA3.1 ERK2). A total of 2 x 106 H9 cells were transfected with 2 µg of plasmid using Amaxa Nucleofection Technology (Cologne, Germany). Transfection efficiency was approximately 58% as determined using green fluorescent protein, and maximal levels of protein expression were observed between 72 and 96 hours.
Measurement of Interferon-γ Production
A total of 2 x 104 leukemic cells (H9, H9rARAC100, Molt-4, and Molt-4rARAC100) were cocultured with 2 x 105 NK cells for 24 hours. Natural killer cells alone or leukemic cells were used as control. Supernatants were collected and tested for production of interferon-γ (IFN-γ). The amounts of IFN-γ were determined using the Quantikine Human IFN-γ ELISA kit (R&D Systems) according to the manufacturer's protocol.
Statistics
Values presented are the mean ± SD of at least three experiments. Comparisons between two groups were performed using Student's t test. P values <.05 were considered to be significant.
Results
Viability of Leukemic Cells on araC Treatment
MTT assay was performed to determine the viability of the leukemic cell lines used on treatment with araC. Cytotoxic effects of araC, expressed as concentrations inhibiting 50% of cell growth (IC50), were decreased in araC-resistant cells when compared with parental cells. Resistance indexes (RIs; ratio of IC50 in H9rARAC100 and H9 cells as well as in Molt-4rARAC100 and Molt-4 cells) of araC for H9 and Molt-4 cells were 2.2 x 104 and 4.3 x 104, respectively (Table 1).
Table 1.
Cytotoxic Effects of araC in Molt-4, Molt-4rARAC100, H9, and H9rARAC100 Cells.
| Cells | IC50 (µM)* | RI† | |
| Without PD98059 | With PD98059 | ||
| Molt-4 | 0.029 ± 0.0018 | 0.024 ± 0.0016 | 4.3 x 104 |
| Molt-4rARAC100 | 1256 ± 234 | 1450 ± 195 | |
| H9 | 0.034 ± 0.0047 | 0.031 ± 0.0051 | 2.2 x 104 |
| H9rARAC100 | 756.8 ± 53.7 | 695.6 ± 78.9 | |
PD98059 was added at a concentration of 20 µM.
Results represent mean values ± SD of three different experiments.
RI (ratio IC50 resistant, IC50 parental cell lines without PD98059).
Cytotoxic Activity of NK Cells Against Leukemic Cell Lines
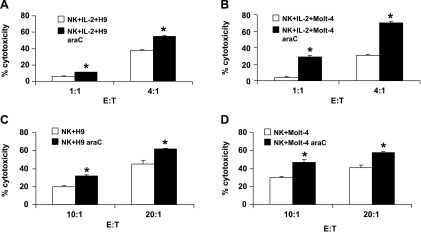
The cytolytic activities of IL-2-activated NK cells against parental as well as H9rARAC100 and Molt-4rARAC100 cell lines were determined. The results show that NK cells effectively kill parental H9 and Molt-4 cell lines. Both araC-resistant cell lines showed higher sensitivity to NK cell lysis than parental cell lines (11% and 55% at E/T 1:1 and 4:1, respectively, for H9rARAC100 vs 6% and 38% at E/T 1:1 and 4:1, respectively, for H9; 29% and 70% at E/T 1:1 and 4:1, respectively, for Molt-4rARAC100 vs 4% and 31% at E/T 1:1 and 4:1, respectively, for Molt-4; Figure 1, A and B). To exclude the direct effects of araC on leukemic cell sensitivity to NK cells, resistant cells used for the experiment were cultured for at least three subcultures without the drug. Similar results were obtained with the cells cultured up to 10 passages without the drug (not shown). Natural killer cell lysis of K562 cell line was used as positive control, and it resulted to approximately 90% lysis (E/T 4:1; not shown). The cytolytic activities of freshly isolated NK cells against parental as well as H9rARAC100 and Molt-4rARAC100 cell lines were also tested. Both araC-resistant cell lines also showed higher sensitivity to NK cell lysis than their parental counterparts. Lysis of leukemic cells was, however, detectable only at higher E/T ratios (32% and 62% at E/T 10:1 and 20:1, respectively, for H9rARAC100 vs 20% and 45% at E/T 10:1 and 20:1, respectively, for H9; 47% and 58% at E/T 10:1 and 20:1, respectively, for Molt-4rARAC100 vs 30% and 41% at E/T 10:1 and 20:1, respectively, for Molt-4; Figure 1, C and D). The production of IFN-γ by NK cells was also measured upon coculture of NK cells with parental as well as H9rARAC100 and Molt-4rARAC100 cell lines. Cocultures of NK cells with parental H9 and Molt-4 cells resulted in 320 ± 25 and 57 ± 12 pg/ml IFN-γ, respectively, for NK/H9 and NK/Molt-4 cocultures. The IFN-γ production was increased 2.25- and 1.7-fold, respectively, in NK/H9rARAC100 and NK/Molt-4rARAC100 cocultures. IL-2-activated NK cells were used for further cytotoxic experiments because low E/T ratios are required.
Figure 1.
Natural killer cell cytotoxicity of parental and araC-resistant leukemic cells. Killing of H9 and H9rARAC100 (H9 araC) as well as Molt-4 and Molt-4rARAC100 (Molt-4 araC) cell lines was assessed by G3PDH release using acella-Tox kit. Five thousand target cells were coincubated with IL-2-activated NK cells (A and B) or freshly isolated NK cells (C and D) at the indicated E/T ratios for 4 hours at 37°C. The results are mean ± SD of three independent experiments. *P < .05 compared with parental control.
Expression of NK Cell-Activating and -Inhibitory Ligands on Surface of Leukemic Cells
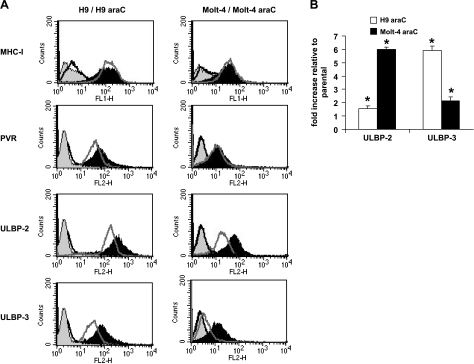
The expression pattern of ligands for the NK cell-activating/inhibitory receptor on the surface of both parental and araC-resistant cell lines was investigated to show whether it correlates with the lysis of leukemic cells by NK cells. Results obtained show that increased NK cell lysis of leukemic cells was associated with a corresponding increase in the cell surface expression of ULBP-2, ULBP-3, and PVR in H9rARAC100 (relative fluorescent units: 380 vs 217 for ULBP-2, 122 vs 50 for ULBP-3, and 43 vs 27 for PVR; Figure 2A). In Molt-4rARAC100 cells, ULBP-2 and ULBP-3 cell surface expression were highly increased, whereas no change was observed for PVR (relative fluorescent units: 82 vs 29 for ULBP-2, 31 vs 6 for ULBP-3, and 10 vs 8 for PVR; Figure 2A). There was no significant change in MHC-I expression level in both parental and araC-resistant cell lines. Both parental and araC-resistant leukemic cell lines did not express MICA/B or ULBP-1 except for Molt-4, which showed minimal expression of ULBP-1 (not shown). The mRNA expression of araC-resistant and parental cell lines used for this study was also evaluated using real-time PCR. As shown in Figure 2B, an increased ULBP-2 and ULBP-3 mRNA expression was observed in araC-resistant cell lines (approximately two-fold ULBP-2, six-fold ULBP-3 increase for H9rARAC100 and six-fold ULBP-2, two-fold ULBP-3 for Molt-4rARAC100) in comparison to their respective parental cell lines. This indicates that araC modifies the NK cell-activating receptor ligand expression at the transcriptional level. The effect of this modification seems to be maintained because several passaging of leukemic cells continued to display this effect (not shown).
Figure 2.
Expression of ligands for NK cell receptors in leukemic cells. (A) Flow cytometric analysis for the expression of ligands in H9 and H9rARAC100 cells and Molt-4 and Molt-4rARAC100 cells. Black line shows isotype control staining, and black-filled histogram shows indicated antibody staining for both H9rARAC100 and Molt-4rARAC100 cell lines. Gray-filled histograms shows isotype control staining, and gray line shows indicated antibody staining for both H9 and Molt-4 cell lines. One representative of at least three separate experiments is shown. (B) Real-time reverse transcription-PCR for the mRNA expression levels of ULBP-2 and ULBP-3 transcripts. Data are expressed as mRNA fold increase in H9rARAC100 and Molt-4rARAC100 cells relative to parental H9 and Molt-4 cells. Histograms are representative of three different experiments. Each experiment was run in duplicate; error bars indicate ± SD. *P < .05 compared with parental control.
NK Cell Recognition of Leukemic Cell Lines Through NKG2D
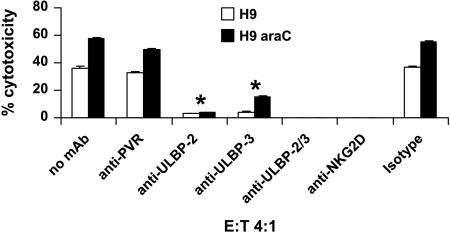
In an attempt to understand a possible mechanism of the increased NK cell lysis of resistant leukemic cells as well as the functionality of NKG2D ligands, mAb masking experiments were performed. As shown in Figure 3, blocking PVR on H9 and H9rARAC100 cells did not show significant decrease in NK cell lysis (36% vs 33% and 58% vs 50%, respectively for H9 and H9rARAC100). However, blocking ULBP-2 or ULBP-3 alone showed a strong inhibition of NK cell lysis (36% vs 3% ULBP-2 and 4% ULBP-3, 58% vs 4% ULBP-2 and 15% ULBP-3, respectively, for H9 and H9rARAC100). In both H9 and H9rARAC100 cell lines, the expression level of ULBP-2 and ULBP-3 was approximately eight- and two-fold higher, respectively, than that of PVR. Combined blocking of ULBP-2 and ULBP-3 on H9rARAC100 cells resulted in total abrogation of NK cell lysis. A 100% inhibition of NK cell lysis was also observed on blocking NKG2D on NK cells (Figure 3). Similar results were obtained using Molt-4 and Molt-4rARAC100 cell lines (not shown). The results suggest that NK cell activation through NKG2D receptor-ligand binding is the possible mechanism involved in the increased lysis of H9rARAC100 and Molt-4rARAC100 cell lines.
Figure 3.
Natural killer cells recognize leukemic cells through NKG2D receptor. Five thousand H9 and H9rARAC100 cells were coincubated with IL-2-activated NK cells at an E/T ratio of 4:1 for 4 hours at 37°C either in the absence or in the presence of mAb. IgG isotype control was used as negative control. The results are mean ± SD of three independent experiments. *P < .05 compared with cells not blocked with mAb.
Possible Mechanism of Increased Ligand Expression in araC-Resistant Leukemic Cells
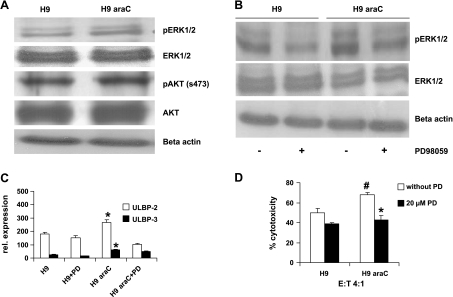
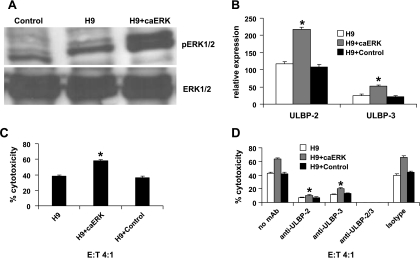
Extracellular signal-regulated kinase and AKT may influence sensitivity of leukemic cells to araC [39–41]. Moreover, both ERK and AKT were shown to be involved in the regulation of expression of NKG2D ligands [42,43]. For these reasons, both ERK and AKT signaling pathways were studied for their constitutive activation status in both parental and araC-resistant cells. Western blot analysis using parental H9 as well as H9rARAC100 cells showed a stronger constitutive phosphorylated ERK but not AKT in H9rARAC100 cells (Figure 4A). To verify the role of ERK signaling pathway in NKG2D ligand expression, flow cytometric analysis and cytotoxicity assay were performed on ERK inhibition of parental H9 and H9rARAC100 cells using 20 µM PD98059. Treatment of both parental and araC-resistant leukemic cell lines with PD98059 shows only minimal effect on cell viability (Table 1). It, however, suppressed their ERK activation status, decreased ULBP-2 (relative expression from 182 to 152 and 266 to 102 for H9 and H9rARAC100, respectively) and ULBP-3 (relative expression from 24 to 14 and 60 to 49 for H9 and H9rARAC100, respectively) surface expression and impaired NK cell lysis (from 50% to 39% and 68% to 43% for H9 and H9rARAC100, respectively; Figure 4, B–D). These results were further confirmed by transfecting parental H9 cells with constitutively active ERK (Figure 5). Transfection of parental H9 cells with constitutively active ERK resulted to 1.9- and 2.1-fold higher ULBP-2 and ULBP-3 ligand expression, respectively, when compared with parental H9 cells (Figure 5B). It also enhanced NK cell lysis by 1.52-fold compared with parental H9 cells (Figure 5C). Furthermore, blocking ULBP-2 and ULBP-3 with mAb inhibited NK cell lysis (Figure 5D). Taken together, these results demonstrate that increased sensitivity of araC-resistant leukemic cells to NK cell lysis is caused by higher NKG2D ligand expression, resulting from more active ERK but not AKT signaling pathway.
Figure 4.
Mechanism of increased ligand expression in araC-resistant cells. (A) The same amount of cell extracts prepared from the same passage of H9 and H9rARAC100 cell cultures was used for Western blot analyses. Constitutive AKT and ERK activation was assessed using antibodies that recognize AKT phosphorylated at ser-473 and ERK1/2 phosphorylated at Thr202/Tyr204. (B) Western blot analysis of H9 and H9rARAC100 cells after specific inhibition of ERK activation using PD98059. Data are representative of at least three experiments. (C) Flow cytometric analysis for ULBP-2 and ULBP-3 expression in H9 and H9rARAC100 cells after a 48-hour treatment with PD98059 (PD). Results are representative of three different experiments. Error bars indicate ±SD, *P < .05 compared with parental H9 cell line. (D) IL-2-activated NK cell cytotoxicty of H9 and H9rARAC100 cells after a 48-hour treatment with PD98059. Results are representative of three different experiments. Error bars indicate ±SD, *P < .05 compared with H9rARAC100 cell line without PD. #P < .05 compared with parental H9 cell line without PD.
Figure 5.
ERK activation is responsible for increased NKG2D ligand expression. (A) ERK activation was assessed by Western blot analysis of constitutively active ERK (caERK)-transfected H9. Empty vector-transfected H9 cells were used as control. (B) Flow cytometric analysis for the expression of NKG2D ligands after transfection of H9 cells with caERK. Results are representative of three different experiments. Error bars indicate ±SD, *P < .05 compared with parental H9 cells (C) NK cell cytotoxicity experiments were performed after transfection of H9 cells with caERK. Results are representative of three different experiments. Error bars indicate ±SD, *P < .05 compared with parental H9 cells. (D) mAb blocking experiments were performed to demonstrate the role of ULBP-2 and ULBP-3 in NK cell lysis after transfection of H9 cells with caERK. Results are representative of three different experiments. Error bars indicate ±SD, *P < .05 compared with cells not blocked with mAb.
Discussion
Numerous experimental studies demonstrated that drug exposure may induce not only resistance but also change other properties of tumor cells which may be related to tumor growth, invasiveness, and immunogenicity [36,44–47]. The present study shows that resistance of T-cell leukemia cell lines to araC is associated with increased sensitivity to NK cell-mediated lysis. These effects were not caused by direct activity of araC on cell metabolism but rather by selection of cell population with altered susceptibility to NK cells because the resistant cells cultured for at least three passages in a medium without the drug were lysed by NK cells to a greater extent than the parental cell population.
Selection of chemotherapy-resistant tumor cells may be associated with increased immunogenicity. Although increased immunogenicity of malignant cells resistant to chemotherapeutic agents has been reported previously [19,36], mechanisms responsible for such effects remain poorly understood. This work focuses on the role of specific ligands for NK cell-activating receptors in the susceptibility of parental and araC-resistant leukemic cells to NK cell lysis. H9 and Molt-4 cells express NKG2D ligands (particularly ULBP-2 and ULBP-3) in addition to PVR (ligand for NK cell-coactivating receptor DNAM-1), whereas they do not express other NKG2D ligands including MICA and MICB. This is in concordance with the hypothesis by Pende et al. [48] stating that most T-cell leukemia cell lines are characterized by a MICA-ULBP+ phenotype. The results, with particular reference to the blocking experiments, show that increased expression of ULBP-2 and ULBP-3 rendered araC-resistant leukemic cell lines to become more sensitive to NK cell lysis. Blocking PVR with mAb could not inhibit NK cell lysis, suggesting that DNAM-1 is not involved in NK cell lysis of H9 and Molt-4 cell lines. The increased NK cell lysis of H9rARAC100 and Molt-4rARAC100 cells could still be observed despite expression of MHC-I molecules on their surface. These findings suggest that ULBP-NKG2D interaction is a major determinant for susceptibility of H9 and Molt-4 cell lines to NK cell lysis.
The expression of NK cell-activating receptor ligands, especially NKG2D ligands, has been shown to be associated with malignant transformation, whereas they are generally only transiently expressed on healthy tissues [49]. Recent experiments demonstrated that NKG2D/NKG2D ligand system stimulates immune surveillance of tumors [50–52]. The capacity of the NKG2D ligand-expressing tumor cell lines to stimulate tumor immunity in vivo was critically dependent on the expression levels of NKG2D ligands on the tumor cell surface. In previous studies, NKG2D ligand surface expression led to increased susceptibility of malignant or virus-infected cells to NK cell- or T cell-mediated lysis [27,28]. Because NK cell activity is guided by a balance of activating and inhibitory signals, and an enhanced NKG2D ligand expression is able to trigger NK cells overcoming inhibitory signals by MHC-I molecules [25,27], even modest changes in NKG2D ligand expression may critically influence NK cell cytotoxic potential. The significance of NKG2D signaling in protection against infection and tumorigenesis is highlighted by the development of mechanisms by viruses and tumor cells to evade detection by this system. Independent reports by Oppenheim et al. [53], Wiemann et al. [54], and Coudert et al. [55], described the down-modulation and altered function of NKG2D in immune effector cells after chronic exposure to its ligands on tumor cells. Therefore, in addition to being involved in increased protection against tumor progression, enhanced expression of NKG2D ligands on surface of araC-resistant cells may also contribute to the evasion of leukemic cells from NK cell reactions during chronic exposure of NKG2D on NK cell surface to NKG2D ligands expressed on the surface of leukemic cells.
Several mechanisms of resistance to araC, such as increased inactivation of araC by cytidine deaminase, decreased intracellular permeation, decreased cellular activation by deoxycytidine kinase, increased degradation of araC nucleotides by 5′-nucleotidase, imbalance of cellular deoxynucleotide pools, increased capability of repair of damaged DNA, and decreased expression of human equilibrative nucleoside transporter 1 (hENT1) involved in the transport of araC across the cell membrane, mutations of hENT1, and deoxycytidine kinase genes have been reported [11–13,56–64]. Moreover, cell regulatory pathways such as ERK or AKT may influence the sensitivity of leukemic cells to araC [39–41]. Treatment of leukemic cells with inhibitors of AKT or ERK pathways was shown to increase their sensitivity to araC treatment [39–41]. Experiments performed in this study show both AKT and ERK to be constitutively activated in H9 cells, with increased activation of ERK but not AKT in resistant cells relative to their parental counterparts. Treatment of leukemic cells with pharmacological inhibitor of ERK showed no toxicity to the cells and did not significantly influence activity of araC in resistant cells. However, NKG2D ligand expression in parental and araC-resistant leukemic cells and their corresponding lysis by NK cells were suppressed. These findings demonstrate that ERK activation is not a resistance mechanism in Molt-4 or H9 cells but is involved in the sensitivity of resistant cells to NK cell lysis.
Extracellular signal-regulated kinase was also shown to increase the expression of NKG2D ligands and the sensitivity to NK cell-mediated lysis of transformed cells derived from solid tumors and leukemia [42]. In fact, Borchers et al. [42] reported the involvement of ERK signaling in the induction of surface expression of NKG2D ligands in human tumor cells after H2O2-induced oxidative stress. The results presented in this study demonstrate that ERK activation is associated with increased ULBP-2 and ULBP-3 expression resulting in enhanced sensitivity of araC-resistant leukemic cells to NK cell lysis. The increased ULBP-2/ULBP-3 ligand expression in constitutively active ERK-transfected H9 cells and the corresponding enhanced NK cell lysis provide direct evidence for the involvement of ERK activation in NKG2D ligand expression in leukemic cells.
In conclusion, this report shows for the first time that T-cell leukemia cell lines that received araC treatment and became resistant are more sensitive to NK cell attack than their parental counterparts. It is possible that part of the efficacy of some chemotherapies and radiotherapies, most of which activate the DNA damage response [65,66], is due to the induction of NKG2D ligands, which, consequently, enhances sensitivity of the cell to the immune system. The present data suggest that development of resistance to chemotherapeutic drugs may be associated with increased immunogenicity of tumor cells. Because such changes persist after cessation of treatment, it is of interest to show whether emergence of chemotherapy-resistant tumor clones with increased sensitivity to NK cell lysis also appear in patients who become refractory to common treatments with araC or other chemotherapeutic agents. The pathway leading to the up-regulation of NKG2D ligands looks promising in the search of targets for design of therapeutic agents to enhance the immunogenicity of transformed cells while reducing overall toxicity.
Acknowledgments
The authors thank Elena Brandi-Barbarito and Rosy Schmidt for their excellent technical assistance.
Abbreviations
- AraC
cytarabine; 1-β-d-arabinofuranosylcytosine
- E/T
effector-target
- ERK
extracellular signal-regulated kinase
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- IL-2
interleukin-2
- IMDM
Iscove's modified Dulbecco's medium
- MHC-I
major histocompatibility complex class I
- MICA/B
MHC-I-related chain A/B
- NK
natural killer
- NKG2D
natural killer group 2D
- ULBP
UL-16 binding protein
Footnotes
The authors gratefully acknowledge the support by the organization “Hilfe für krebskranke Kinder, Frankfurt/Main e.V.,” by the foundation “Frankfurter Stiftung für krebskranke Kinder,” and by the European Commission-funded Co-operative Research and Specific Targeted Research Projects: COOP-CT-2004, contract no. 512864 and LSHB-CT-2004, contract no. 512054 respectively.
References
- 1.Cheson BD. New antimetabolites in the treatment of human malignancies. Semin Oncol. 1992;19:695–706. [PubMed] [Google Scholar]
- 2.Robak T. Purine nucleoside analogues in the treatment of myleoid leukemias. Leuk Lymphoma. 2003;44:391–409. doi: 10.1080/1042819021000035608. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq E. New developments in anti-HIV chemotherapy. Curr Med Chem. 2001;8:1543–1572. doi: 10.2174/0929867013371842. [DOI] [PubMed] [Google Scholar]
- 4.van Laar JA, Rustum YM, Ackland SP, van Groeningen CJ, Peters GJ. Comparison of 5-fluoro-2′-deoxyuridine with 5-fluorouracil and their role in the treatment of colorectal cancer. Eur J Cancer. 1998;34:296–306. doi: 10.1016/s0959-8049(97)00366-3. [DOI] [PubMed] [Google Scholar]
- 5.Keating MJ, McCredie KB, Bodey GP, Smith TL, Gehan E, Freireich EJ. Improved prospects for long-term survival in adults with acute myelogenous leukemia. JAMA. 1982;248:2481–2486. [PubMed] [Google Scholar]
- 6.Rustum YM, Preisler HD. Correlation between leukemic cell retention of 1-beta-d-arabinofuranosylcytosine 5′-triphosphate and response to therapy. Cancer Res. 1979;39:42–49. [PubMed] [Google Scholar]
- 7.Tedeschi A, Montillo M, Strocchi E, Cafro AM, Tresoldi E, Intropido L, Nichelatti M, Marbello L, Baratè C, Camaggi CM, et al. High-dose idarubicin in combination with Ara-C in patients with relapsed or refractory acute lymphoblastic leukemia: a pharmacokinetic and clinical study. Cancer Chemother Pharmacol. 2007;59:771–779. doi: 10.1007/s00280-006-0332-4. [DOI] [PubMed] [Google Scholar]
- 8.Candoni A, Michelutti A, Simeone E, Damiani D, Baccarani M, Fanin R. Efficacy of liposomal daunorubicin and cytarabine as reinduction chemotherapy in relapsed acute lymphoblastic leukaemia despite expression of multidrug resistance-related proteins. Eur J Haematol. 2006;77:293–299. doi: 10.1111/j.1600-0609.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 9.Giebel S, Krawczyk-Kulis M, Adamczyk-Cioch M, Jakubas B, Palynyczko G, Lewandowski K, Dmoszynska A, Skotnicki A, Nowak K, Holowiecki J, et al. Fludarabine, cytarabine, and mitoxantrone (FLAM) for the treatment of relapsed and refractory adult acute lymphoblastic leukemia. A phase study by the Polish Adult Leukemia Group (PALG) Ann Hematol. 2006;85:717–722. doi: 10.1007/s00277-006-0121-5. [DOI] [PubMed] [Google Scholar]
- 10.Cadman E, Farber L, Berd D, Bertino J. Combination therapy for diffuse histiocytic lymphoma that includes antimetabolites. Cancer Treat Rep. 1977;61:1109–1116. [PubMed] [Google Scholar]
- 11.Kaspers GJ, Zwaan CM, Veerman AJ, Rots MG, Pieters R, Bucsky P, Domula M, Gobel U, Graf N, Havers W, et al. Cellular drug resistance in acute myeloid leukemia: literature review and preliminary analysis of an ongoing collaborative study. Klin Padiatr. 1999;211:239–244. doi: 10.1055/s-2008-1043795. [DOI] [PubMed] [Google Scholar]
- 12.Yamada S, Hongo T, Okada S, Watanabe C, Fujii Y, Ohzeki T. Clinical relevance of in vitro chemoresistance in childhood acute myeloid leukemia. Leukemia. 2001;15:1892–1897. doi: 10.1038/sj.leu.2402305. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar M, Han T, Damaraju V, Carpenter P, Cass CE, Agarwal RP. Cytosine arabinoside affects multiple cellular factors and induces drug resistance in human lymphoid cells. Biochem Pharmacol. 2005;70:426–432. doi: 10.1016/j.bcp.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Debatin KM. Cytotoxic drugs, programmed cell death, and the immune system: defining new roles in an old play. J Natl Cancer Inst. 1997;89:750–751. doi: 10.1093/jnci/89.11.750. [DOI] [PubMed] [Google Scholar]
- 15.Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 16.Micheau O, Solary E, Hammann A, Martin F, Dimanche-Boitrel MT. Sensitization of cancer cells treated with cytotoxic drugs to fas-mediated cytotoxicity. J Natl Cancer Inst. 1997;89:783–789. doi: 10.1093/jnci/89.11.783. [DOI] [PubMed] [Google Scholar]
- 17.Classen CF, Fulda S, Friesen C, Debatin KM. Decreased sensitivity of drug-resistant cells towards T cell cytotoxicity. Leukemia. 1999;13:410–418. doi: 10.1038/sj.leu.2401335. [DOI] [PubMed] [Google Scholar]
- 18.Friesen C, Fulda S, Debatin KM. Deficient activation of the CD95 (APO-1/Fas) system in drug-resistant cells. Leukemia. 1997;11:1833–1841. doi: 10.1038/sj.leu.2400827. [DOI] [PubMed] [Google Scholar]
- 19.Posovszky C, Friesen C, Herr I, Debatin KM. Chemotherapeutic drugs sensitize pre-B ALL cells for CD95- and cytotoxic T-lymphocyte-mediated apoptosis. Leukemia. 1999;13:400–409. doi: 10.1038/sj.leu.2401327. [DOI] [PubMed] [Google Scholar]
- 20.Classen CF, Falk CS, Friesen C, Fulda S, Herr I, Debatin KM. Natural killer resistance of a drug-resistant leukemia cell line, mediated by upregulation of HLA class I expression. Haematologica. 2003;88:509–521. [PubMed] [Google Scholar]
- 21.Treichel RS, Bunuan M, Hahn N, Wee K. Altered conjugate formation and altered apoptosis of multidrug-resistant human leukemia cell line affects susceptibility to killing by activated natural killer (NK) cells. Int J Cancer. 2004;108:78–85. doi: 10.1002/ijc.11555. [DOI] [PubMed] [Google Scholar]
- 22.Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Human natural killer cells: molecular mechanisms controlling NK cell activation and tumor cell lysis. Immunol Lett. 2005;100:7–13. doi: 10.1016/j.imlet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 24.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 25.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 26.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 27.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novelMHCclass I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 28.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 29.Kubin M, Cassiano L, Chalupny J, Chin W, Cosman D, Fanslow W, Mullberg J, Rousseau AM, Ulrich D, Armitage R. ULBP1, 2, 3: novel MHC class I-related molecules that bind to human cytomegalovirus glycoprotein UL16, activate NK cells. Eur J Immunol. 2001;31:1428–1437. doi: 10.1002/1521-4141(200105)31:5<1428::AID-IMMU1428>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland CL, Chalupny NJ, Schooley K, VandenBos T, Kubin M, Cosman D. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J Immunol. 2002;168:671–679. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- 31.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4:557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 32.Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16:533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 33.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 35.Castriconi R, Dondero A, Corrias MV, Lanino E, Pende D, Moretta L, Bottino C, Moretta A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 36.Ogbomo H, Hahn A, Geiler J, Michaelis M, Doerr HW, Cinatl J., Jr NK sensitivity of neuroblastoma cells determined by a highly sensitive coupled luminescent method. Biochem Biophys Res Commun. 2006;339:375–379. doi: 10.1016/j.bbrc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 37.Guimarães-Ferreira CA, Rodrigues EG, Mortara RA, Cabral H, Serrano FA, Ribeiro-dos-Santos R, Travassos LR. Antitumor effects in vitro and in vivo and mechanisms of protection against melanoma B16F10-Nex2 cells by fastuosain, a cysteine proteinase from Bromelia fastuosa. Neoplasia. 2007;9:723–733. doi: 10.1593/neo.07427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaheta RA, Weich E, Marian D, Bereiter-Hahn J, Jones J, Jonas D, Michaelis M, Doerr HW, Cinatl J., Jr Human cytomegalovirus infection alters PC3 prostate carcinoma cell adhesion to endothelial cells and extracellular matrix. Neoplasia. 2006;8:807–816. doi: 10.1593/neo.06379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bortul R, Tazzari PL, Billi AM, Tabellini G, Mantovani I, Cappellini A, Grafone T, Martinelli G, Conte R, Martelli AM. Deguelin, a PI3K/AKT inhibitor, enhances chemosensitivity of leukaemia cells with an active PI3K/AKT pathway. Br J Haematol. 2005;129:677–686. doi: 10.1111/j.1365-2141.2005.05504.x. [DOI] [PubMed] [Google Scholar]
- 40.Grandage VL, Gale RE, Linch DC, Khwaja A. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kappaB, MAPkinase and p53 pathways. Leukemia. 2005;19:586–594. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- 41.Yin B, Morgan K, Hasz DE, Mao Z, Largaespada DA. Nfl gene inactivation in acute myeloid leukemia cells confers cytarabine resistance through MAPK and mTOR pathways. Leukemia. 2006;20:151–154. doi: 10.1038/sj.leu.2404033. [DOI] [PubMed] [Google Scholar]
- 42.Borchers MT, Harris NL, Wesselkamper SC, Vitucci M, Cosman D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L222–L231. doi: 10.1152/ajplung.00327.2005. [DOI] [PubMed] [Google Scholar]
- 43.Boissel N, Rea D, Tieng V, Dulphy N, Brun M, Cayuela JM, Rousselot P, Tamouza R, Le Bouteiller P, Mahon FX, et al. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J Immunol. 2006;176:5108–5116. doi: 10.4049/jimmunol.176.8.5108. [DOI] [PubMed] [Google Scholar]
- 44.Kotchetkov R, Cinatl J, Blaheta R, Vogel JU, Karaskova J, Squire J, Hernaiz Driever P, Klingebiel T, Cinatl J., Jr Development of resistance to vin-cristine and doxorubicin in neuroblastoma alters malignant properties and induces additional karyotype changes: a preclinical model. Int J Cancer. 2003;104:36–43. doi: 10.1002/ijc.10917. [DOI] [PubMed] [Google Scholar]
- 45.Liang Y, O'Driscoll L, McDonnell S, Doolan P, Oglesby I, Duffy K, O'Connor R, Clynes M. Enhanced in vitro invasiveness and drug resistance with altered gene expression patterns in a human lung carcinoma cell line after pulse selection with anticancer drugs. Int J Cancer. 2004;111:484–493. doi: 10.1002/ijc.20230. [DOI] [PubMed] [Google Scholar]
- 46.Glynn SA, Gammell P, Heenan M, O'Connor R, Liang Y, Keenan J, Clynes M. A new superinvasive in vitro phenotype induced by selection of human breast carcinoma cells with the chemotherapeutic drugs paclitaxel and doxorubicin. Br J Cancer. 2004;91:1800–1807. doi: 10.1038/sj.bjc.6602221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groschel B, Kaufmann A, Cinatl J, Doerr HW, Cinatl J., Jr Cytarabine treatment of human T-lymphoid cells induces decreased HIV-1 receptor expression and reduced HIV-1 susceptibility. Nucleosides Nucleotides Nucleic Acids. 2001;20:1433–1437. doi: 10.1081/NCN-100002571. [DOI] [PubMed] [Google Scholar]
- 48.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 49.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a newparadigm in immune recognition? Curr Opin Immunol. 2002;14:306–311. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 50.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lanier LL. A renaissance for the tumor immunosurveillance hypothesis. Nat Med. 2001;7:1178–1180. doi: 10.1038/nm1101-1178. [DOI] [PubMed] [Google Scholar]
- 52.Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, Nanni M, Biassoni R, Bottino C, Moretta A, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::aid-immu1076>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 53.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 54.Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 55.Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, Held W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–1717. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 56.Wiley JS, Jones SP, Sawyer WH, Paterson AR. Cytosine arabinoside influx and nucleoside transport sites in acute leukemia. J Clin Invest. 1982;69:479–489. doi: 10.1172/JCI110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steuart CD, Burke PJ. Cytidine deaminase and the development of resistance to arabinosyl cytosine. Nat New Biol. 1971;233:109–110. doi: 10.1038/newbio233109a0. [DOI] [PubMed] [Google Scholar]
- 58.Kessel D, Hall TC, Rosenthal D. Uptake and phosphorylation of cytosine arabinoside by normal and leukemic human blood cells in vitro. Cancer Res. 1969;29:459–463. [PubMed] [Google Scholar]
- 59.Tattersall MH, Ganeshaguru K, Hoffbrand AV. Mechanisms of resistance of human acute leukaemia cells to cytosine arabinoside. Br J Haematol. 1974;27:39–46. doi: 10.1111/j.1365-2141.1974.tb06772.x. [DOI] [PubMed] [Google Scholar]
- 60.Barret JM, Hill BT. DNA repair mechanisms associated with cellular resistance to antitumor drugs: potential novel targets. Anticancer Drugs. 1998;9:105–123. doi: 10.1097/00001813-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Gandhi V, Estey E, Keating MJ, Chucrallah A, Plunkett W. Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood. 1996;87:256–264. [PubMed] [Google Scholar]
- 62.Groschel B, Himmel N, Cinatl J, Perigaud C, Gosselin G, Imbach JL, Doerr HW, Cinatl J., Jr ddC- and 3TC-bis(SATE) monophosphate pro-drugs overcome cellular resistance mechanisms to HIV-1 associated with cytidine kinase deficiency. Nucleosides Nucleotides. 1999;18:921–926. doi: 10.1080/15257779908041600. [DOI] [PubMed] [Google Scholar]
- 63.Hubeek I, Stam RW, Peters GJ, Broekhuizen R, Meijerink JP, van Wering ER, Gibson BE, Creutzig U, Zwaan CM, Cloos J, et al. The human equilibrative nucleoside transporter 1 mediates in vitro cytarabine sensitivity in childhood acute myeloid leukaemia. Br J Cancer. 2005;93:1388–1394. doi: 10.1038/sj.bjc.6602881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai J, Damaraju VL, Groulx N, Mowles D, Peng Y, Robins MJ, Cass CE, Gros P. Two distinct molecular mechanisms underlying cytarabine resistance in human leukemic cells. Cancer Res. 2008;68:2349–2357. doi: 10.1158/0008-5472.CAN-07-5528. [DOI] [PubMed] [Google Scholar]
- 65.Bignami M, Casorelli I, Karran P. Mismatch repair and response to DNA-damaging antitumour therapies. Eur J Cancer. 2003;39:2142–2149. doi: 10.1016/s0959-8049(03)00569-0. [DOI] [PubMed] [Google Scholar]
- 66.Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004;4:216–225. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]