Abstract
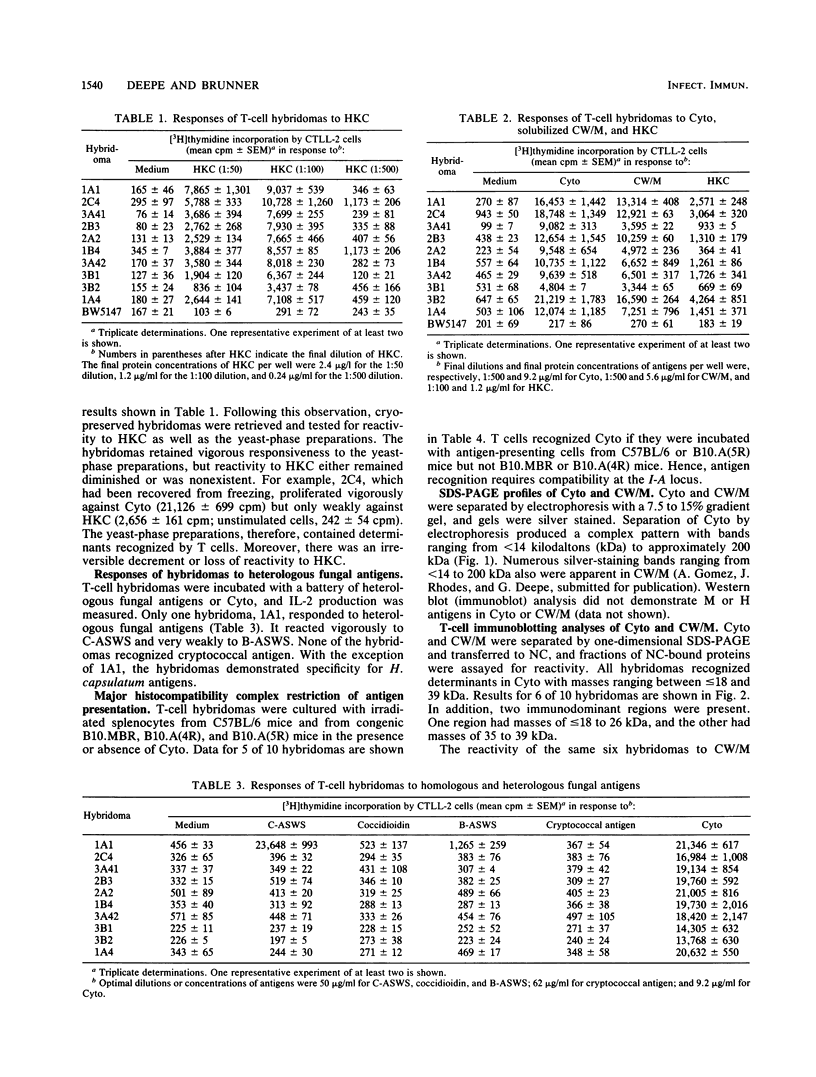
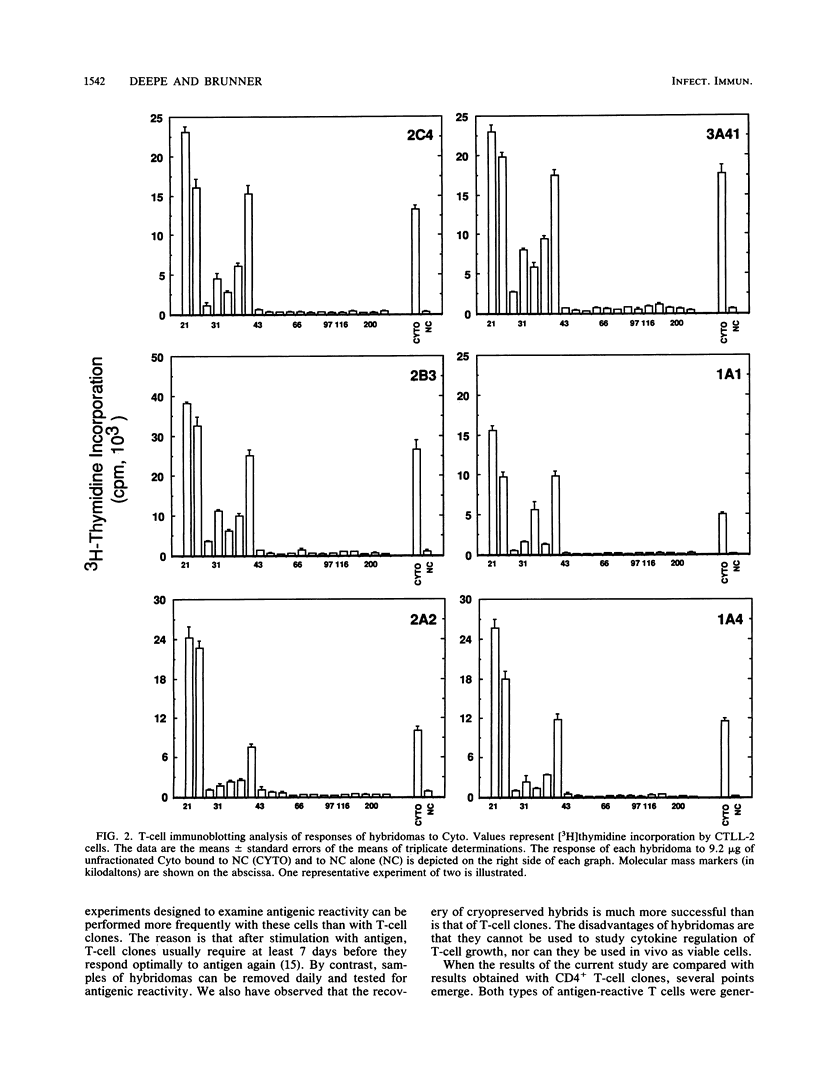
Activation of CD4+ T cells is a crucial step in the elimination of Histoplasma capsulatum yeast cells from tissues. However, only a limited amount of information exists concerning the immunobiology of H. capsulatum-reactive T cells that are CD4+. To facilitate the analysis of the functional activities of this T-cell subpopulation, we developed a panel of 10 murine T-cell hybridomas from splenocytes of immune C57BL/6 mice. All hybridomas reacted with monoclonal anti-CD4+ antibody and released interleukin-2 after stimulation with histoplasmin. Within 3 weeks, the reactivity of hybridomas to histoplasmin declined dramatically, yet the cells responded vigorously to yeast-phase preparations that were enriched for cytosol, cell wall, or cell membrane. Of 10 hybridomas studied, only one recognized heterologous fungal antigens. Responsiveness to yeast-phase antigens was restricted by I-Ab. We mapped determinants in cytosol and cell wall or cell membrane by the technique of one-dimensional T-cell immunoblotting. The patterns of responses of hybridomas to cytosol were nearly uniform. All hybridomas responded to two immunodominant regions in cytosol with masses ranging from less than or equal to 18 to 26 kilodaltons (kDa) and 35 to 39 kDa. All hybridomas tested responded to determinants in the cell wall or cell membrane preparation with masses of 35 to 39 kDa. These hybridomas provide a useful tool for defining yeast-phase antigens that trigger T-cell activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Blackstock R., Hall N. K., Hernandez N. C. Characterization of a suppressor factor that regulates phagocytosis by macrophages in murine cryptococcosis. Infect Immun. 1989 Jun;57(6):1773–1779. doi: 10.1128/iai.57.6.1773-1779.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick E. W., Baum G. L., Furcolow M. L., Huppert M., Kaufman L., Pappagianis R. Scientific Assembly statement. The use of skin tests and serologic tests in histoplasmosis, coccidioidomycosis, and blastomycosis, 1973. Am Rev Respir Dis. 1973 Jul;108(1):156–159. doi: 10.1164/arrd.1973.108.1.156. [DOI] [PubMed] [Google Scholar]
- Cox R. A. Cross-reactivity between antigens of Coccidioides immitis, Histoplasma capsulatum and Blastomyces dermatitidis in lymphocyte transformation assays. Infect Immun. 1979 Sep;25(3):932–938. doi: 10.1128/iai.25.3.932-938.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe G. S., Jr Protective immunity in murine histoplasmosis: functional comparison of adoptively transferred T-cell clones and splenic T cells. Infect Immun. 1988 Sep;56(9):2350–2355. doi: 10.1128/iai.56.9.2350-2355.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Smith J. G., Sonnenfeld G., Denman D., Bullock W. E. Development and characterization of Histoplasma capsulatum-reactive murine T-cell lines and clones. Infect Immun. 1986 Dec;54(3):714–722. doi: 10.1128/iai.54.3.714-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel P. L., Jr, Murphy J. W. Characterization of an in vitro-stimulated, Cryptococcus neoformans-specific second-order suppressor T cell and its precursor. Infect Immun. 1988 May;56(5):1267–1272. doi: 10.1128/iai.56.5.1267-1272.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A. M., Bullock W. E., Taylor C. L., Deepe G. S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988 Jul;56(7):1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. E., Deepe G. S., Jr Characterization of antigenic determinants in histoplasmin that stimulate Histoplasma capsulatum-reactive T cells in vitro. Infect Immun. 1988 Sep;56(9):2343–2349. doi: 10.1128/iai.56.9.2343-2349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melby P. C., Sacks D. L. Identification of antigens recognized by T cells in human leishmaniasis: analysis of T-cell clones by immunoblotting. Infect Immun. 1989 Oct;57(10):2971–2976. doi: 10.1128/iai.57.10.2971-2976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Hancock K., Maddison S. E., Beatty A. L., Moss D. M. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA). J Immunol. 1984 May;132(5):2607–2613. [PubMed] [Google Scholar]
- Von Behren L. A., Chaudhary S., Rabinovich S., Shu M. D., Tewari R. P. Protective effect of poly-2-vinylpyridine-N-oxide on susceptibility of silica-treated mice to experimental histoplasmosis. Infect Immun. 1983 Nov;42(2):818–823. doi: 10.1128/iai.42.2.818-823.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde D. B., Fitch F. W. Antigen-reactive cloned helper T cells. I. Unresponsiveness to antigenic restimulation develops after stimulation of cloned helper T cells. J Immunol. 1984 Apr;132(4):1632–1638. [PubMed] [Google Scholar]
- Williams D. M., Graybill J. R., Drutz D. J. Histoplasma capsulatum infection in nude mice. Infect Immun. 1978 Sep;21(3):973–977. doi: 10.1128/iai.21.3.973-977.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsham P. L., Goldman W. E. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J Med Vet Mycol. 1988 Jun;26(3):137–143. [PubMed] [Google Scholar]
- Wu-Hsieh B. A., Howard D. H. Inhibition of the intracellular growth of Histoplasma capsulatum by recombinant murine gamma interferon. Infect Immun. 1987 Apr;55(4):1014–1016. doi: 10.1128/iai.55.4.1014-1016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol. 1984 May;132(5):2593–2597. [PubMed] [Google Scholar]
- Wu-Hsieh B., Zlotnik A., Howard D. H. T-cell hybridoma-produced lymphokine that activates macrophages to suppress intracellular growth of Histoplasma capsulatum. Infect Immun. 1984 Jan;43(1):380–385. doi: 10.1128/iai.43.1.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]