Abstract
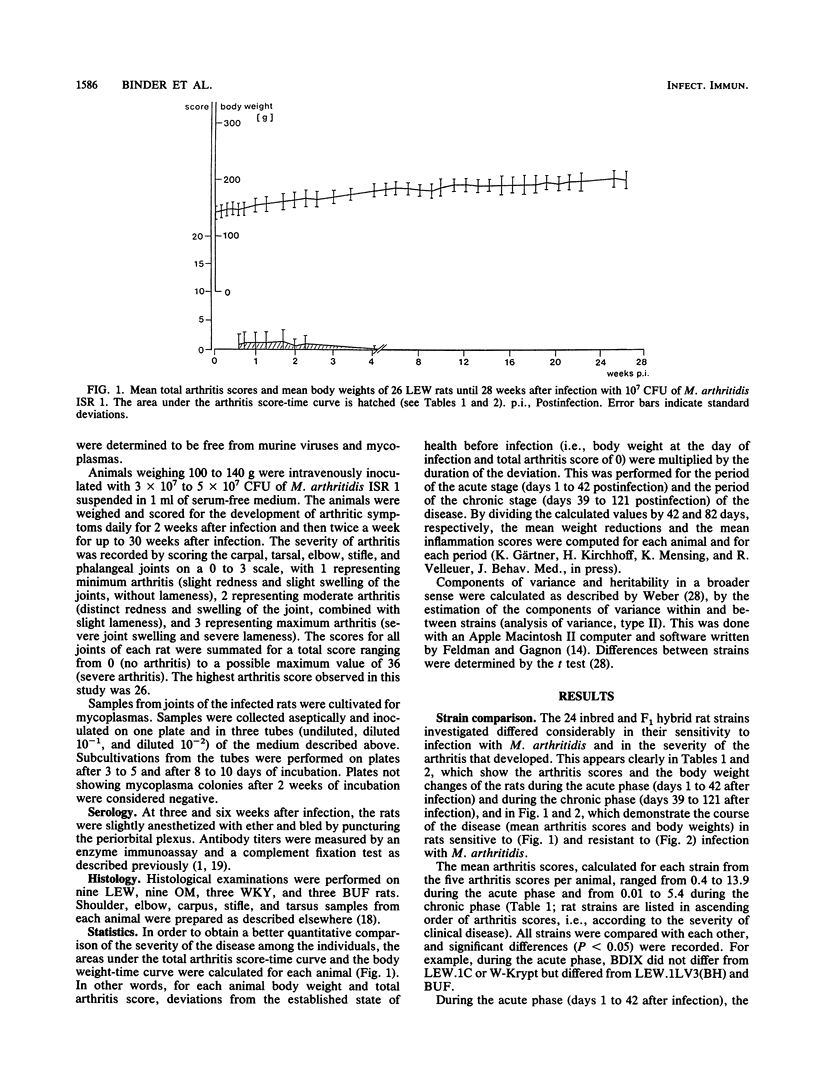
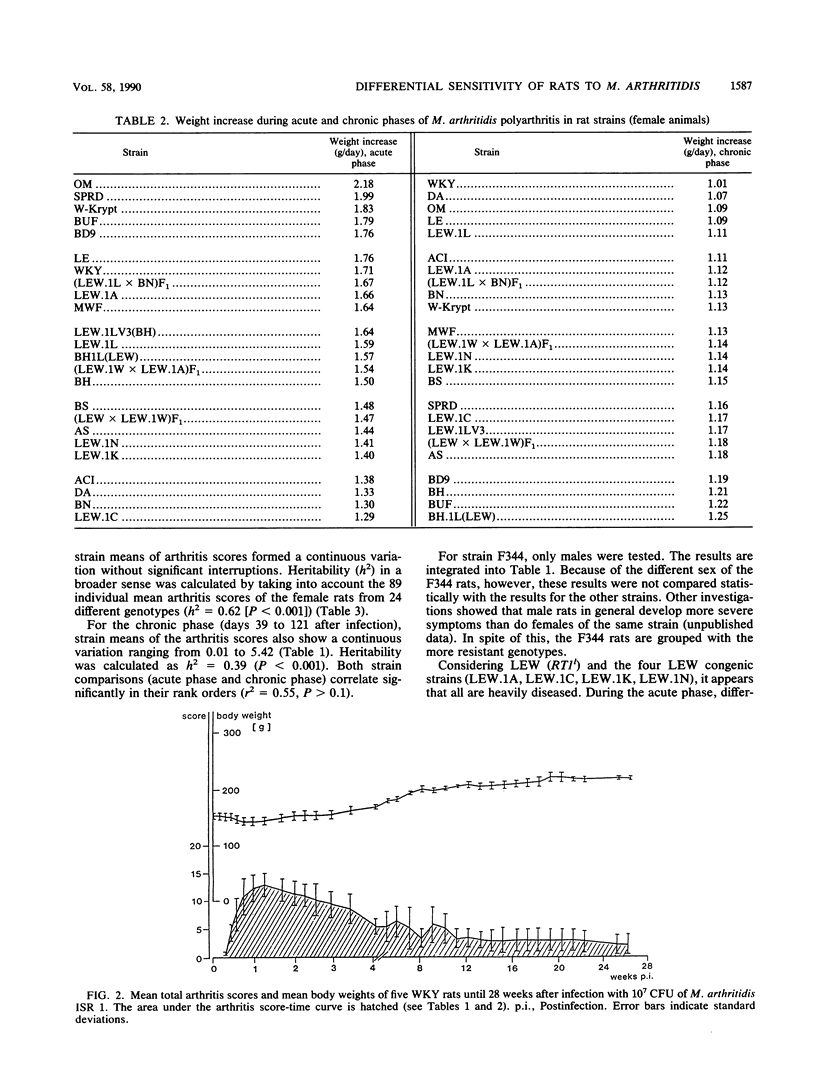
At least 5 female rats from each of 24 inbred (ACI, AS, BDIX, BH, BN, BS, BUF, DA, LE, LEW, MWF, OM, SPRD-Cu3, W-Krypt, and WKY), RT1 congenic [BH.1L(LEW), LEW.1A(AVN), LEW.1C(WIST), LEW.1LV3(BH), LEW.1K(SHR), and LEW.1N(BN)], and F1 hybrid [(LEW x BN)F1, (LEW.1W x LEW.1A)F1, and (LEW x LEW.1W)F1] strains, representing eight independent major histocompatibility complex (MHC) haplotypes (a, b, c, dv1, k, l, n, and u) and five related RT1 haplotypes (av1, lv1, lv3, uv2, and uv3), were inoculated intravenously with Mycoplasma arthritidis, and the severity of the polyarthritis that developed was determined by estimating arthritis scores and weight reductions. The 24 inbred, congenic, and F1 hybrid rat strains differed considerably in their sensitivity to infection with M. arthritidis and in the severity of the polyarthritis that they developed. Statistical evaluation showed that in the acute phase (days 1 to 42 after infection) as well as in the chronic phase (days 39 to 121 after infection) of the disease, the means of the arthritis scores for the strains form a continuous variation without significant interruptions, with the very sensitive LEW rats, the RT1 congenic rats on LEW background, the F1 hybrids with LEW, and the MWF, BS, BH, and DA rats on one end and the resistant WKY, BUF, W-Krypt, LE, and OM rats on the other end. A continuous variation was also observed for the means of the growth rates. There were, however, no significant differences between the sensitive and the resistant rat strains in the antibody titers determined by complement fixation test and enzyme immunoassay. Heritabilities of arthritis scores were calculated for all strains (h2 = 0.39 to 0.62), for the RT1 congenic strains (h2 = 0.04 to 0.14), and for several strains with identical MHC genes (h2 = 0.61 to 0.93). The results show that non-MHC genes are probably responsible for the sensitivity of rats to infection with M. arthritidis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammar A. M., Heitmann J., Kirchhoff H. Untersuchung von Stutenseren auf Antikörper gegen Acholeplasmen und Mykoplasmen mit dem Enzyme Linked Immunosorbent Assay (ELISA). Zentralbl Bakteriol A. 1980;247(4):517–525. [PubMed] [Google Scholar]
- Anderle S. K., Allen J. B., Wilder R. L., Eisenberg R. A., Cromartie W. J., Schwab J. H. Measurement of streptococcal cell wall in tissues of rats resistant or susceptible to cell wall-induced chronic erosive arthritis. Infect Immun. 1985 Sep;49(3):836–837. doi: 10.1128/iai.49.3.836-837.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981 Nov;127(5):1931–1936. [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. III. Ir gene control of lymphocyte transformation correlates with binding of the mitogen to specific Ia-bearing cells. J Immunol. 1982 Oct;129(4):1352–1359. [PubMed] [Google Scholar]
- Cole B. C., Griffiths M. M., Sullivan G. J., Ward J. R. Role of non-RT1 genes in the response of rat lymphocytes to Mycoplasma arthritidis T cell mitogen, concanavalin A and phytohemagglutinin. J Immunol. 1986 Apr 1;136(7):2364–2369. [PubMed] [Google Scholar]
- Cole B. C., Piepkorn M. W., Wright E. C. Influence of genes of the major histocompatibility complex on ulcerative dermal necrosis induced in mice by Mycoplasma arthritidis. J Invest Dermatol. 1985 Oct;85(4):357–361. doi: 10.1111/1523-1747.ep12276973. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Sullivan G. J., Daynes R. A., Sayed I. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. II. Cellular requirements for T cell transformation mediated by a soluble Mycoplasma mitogen. J Immunol. 1982 May;128(5):2013–2018. [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N., Hassell L. A., Washburn L. R., Ward J. R. Toxicity but not arthritogenicity of Mycoplasma arthritidis for mice associates with the haplotype expressed at the major histocompatibility complex. Infect Immun. 1983 Sep;41(3):1010–1015. doi: 10.1128/iai.41.3.1010-1015.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N. I-E/I-C region-associated induction of murine gamma interferon by a haplotype-restricted polyclonal T-cell mitogen derived from Mycoplasma arthritidis. Infect Immun. 1984 Jan;43(1):302–307. doi: 10.1128/iai.43.1.302-307.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Simecka J. W., Williamson J. S., Ross S. E., Juliana M. M., Thorp R. B., Cassell G. H. Nonspecific lymphocyte responses in F344 and LEW rats: susceptibility to murine respiratory mycoplasmosis and examination of cellular basis for strain differences. Infect Immun. 1985 Jul;49(1):152–158. doi: 10.1128/iai.49.1.152-158.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis N. F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975 Jun;27(6):337–339. [PubMed] [Google Scholar]
- Griffiths M. M., DeWitt C. W. Genetic control of collagen-induced arthritis in rats: the immune response to type II collagen among susceptible and resistant strains and evidence for multiple gene control. J Immunol. 1984 Jun;132(6):2830–2836. [PubMed] [Google Scholar]
- Griffiths M. M., DeWitt C. W. Modulation of collagen-induced arthritis in rats by non-RT1-linked genes. J Immunol. 1984 Dec;133(6):3043–3046. [PubMed] [Google Scholar]
- Hermanns W., Schulz L. C., Kirchhoff H., Heitmann J. Studies of polyarthritis caused by mycoplasma arthritidis in rats. III. Histopathological findings. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):423–434. [PubMed] [Google Scholar]
- Kirchhoff H., Ammar A. M., Heitmann J., Dubenkropp H., Schmidt R. Serological investigation of horse sera for antibodies against mycoplasmas and acholeplasmas. Vet Microbiol. 1982 May;7(2):147–156. doi: 10.1016/0378-1135(82)90026-8. [DOI] [PubMed] [Google Scholar]
- Kirchhoff H., Heitmann J., Ammar A., Hermanns W., Schulz L. C. Studies of polyarthritis caused by Mycoplasma arthritidis in rats. I. Detection of the persisting Mycoplasma antigen by the enzyme immune assay (EIA) and conventional culture technique. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Mar;254(1):129–138. [PubMed] [Google Scholar]
- Kirchhoff H., Heitmann J., Mielke H., Dubenkropp H., Schmidt R. Studies of polyarthritis caused by Mycoplasma arthritidis in rats. II. Serological investigation of rats experimentally infected with M. arthritidis ISR 1. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Apr;254(2):275–280. [PubMed] [Google Scholar]
- Laber G., Walzl H., Schütze E. Klinische und histopathologische Befunde bei der Mykoplasma-Polyarthritis der Ratte. I. Der Ablauf der Infektion in den ersten 8 Tagen. Zentralbl Bakteriol Orig A. 1975;230(3):385–397. [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. M., Young W. S., 3rd, Bernardini R., Calogero A. E., Chrousos G. P., Gold P. W., Wilder R. L. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Cole B. C., Gelman M. I., Ward J. R. Chronic arthritis of rabbits induced by mycoplasmas. I. Clinical microbiologic, and histologic features. Arthritis Rheum. 1980 Jul;23(7):825–836. doi: 10.1002/art.1780230709. [DOI] [PubMed] [Google Scholar]
- Washburn L. R., Cole B. C., Ward J. R. Chronic arthritis of rabbits induced by mycoplasmas. III. Induction with nonviable Mycoplasma arthritidis antigens. Arthritis Rheum. 1982 Aug;25(8):937–946. doi: 10.1002/art.1780250805. [DOI] [PubMed] [Google Scholar]
- Wilder R. L., Allen J. B., Wahl L. M., Calandra G. B., Wahl S. M. The pathogenesis of group A streptococcal cell wall-induced polyarthritis in the rat. Comparative studies in arthritis resistant and susceptible inbred rat strains. Arthritis Rheum. 1983 Dec;26(12):1442–1451. doi: 10.1002/art.1780261205. [DOI] [PubMed] [Google Scholar]


