Abstract
Tissues lost as a consequence of periodontal diseases, i.e. bone, cementum and a functional periodontal ligament (PDL), can be restored to some degree. Nevertheless, results are often disappointing. There is a need to develop new paradigms for regenerating periodontal tissues that are based on an understanding of the cellular and molecular mechanisms regulating the development and regeneration of periodontal tissues. As one approach we have developed strategies for maintaining cementoblasts in culture by first determining the gene profile for these cells in situ. Next, cells were immortalized in vitro using SV 40 large T antigen (SV40 Tag) or by using mice containing transgenes enabling cellular immortality in vitro. Cementoblasts in vitro retained expression of genes associated with mineralized tissues, bone sialoprotein and osteocalcin, that were not linked with periodontal fibroblasts either in situ or in vitro. Further, cementoblasts promoted mineralization in vitro as measured by von Kossa and ex vivo using a severely compromised immunodeficient (SCID) mouse model. These cells responded to growth factors by eliciting changes in gene profile and mitogenesis and to osteotropic hormones by evoking changes in gene profile and ability to induce mineral nodule formation in vitro. The ultimate goal of these studies is to provide the knowledge base required for designing improved modalities for use in periodontal regenerative therapies.
Keywords: cementoblasts, mineralization, periodontal regeneration, osteoblasts
An ultimate goal of periodontal regeneration therapy is to predictably restore tissues lost as a consequence of periodontal disease, i.e. bone, cementum and periodontal ligament (PDL). Regeneration of these tissues has to be timed precisely in order to establish a functional periodontium and to prevent fusion of surrounding bone with the tooth root. There is increasing evidence that regeneration of cementum is critical for establishing a functional PDL region. For example, cementum infected with bacterial endotoxins and/or lacking appropriate formation due to metabolic disorders does not allow for PDL insertion into the tooth root (1, 2). Further, Giannobile et al. (3), using a beagle dog periodontal defect model, noted consistently that when the advancing bone front did not outpace the adjacent regenerative cementum formation, ankylosis did not occur.
Therefore, methods to better define the cementoblast are required for designing improved periodontal regenerative therapies. As one approach, gene profiles for cementoblasts and periodontal ligament cells were determined in situ and then confirmed in vitro. Using this process, coupled with the advantage of having “immortalized” mice, cementoblasts and PDL cells have been isolated, partially characterized and maintained in culture. In this paper, various strategies used for obtaining PDL cells and cementoblasts that reflect their respective counterparts in situ are presented. As discussed below, cells expressing bone sialoprotein (BSP) and osteocalcin (OC) elicit a parathyroid hormone (PTH)-mediated cAMP response and promote mineralization, in vitro and ex vivo. In contrast, cells that do not express BSP or OC did not elicit a PTH-mediated cAMP response and lacked the capacity to induce mineral formation, in vitro or ex vivo.
Materials and methods
As discussed below, in order to define the “cementoblast” and establish the function of this cell type during regeneration of periodontal tissues, a strategy was developed to define cementoblasts in situ, in vitro and then ex vivo.
In situ
Using in situ hybridization and imtnunocyto-chemistry, timed and spatial expression of specific proteins were mapped during marine tooth root development: i.e. from d 16 to 60 (4–8). Days are counted from vaginal plug date, which is assigned d 0, and animals are usually born on d 19. Proteins examined included BSP. OC, osteopontin (OPN), and Types I and XII collagen.
In vitro cell isolation
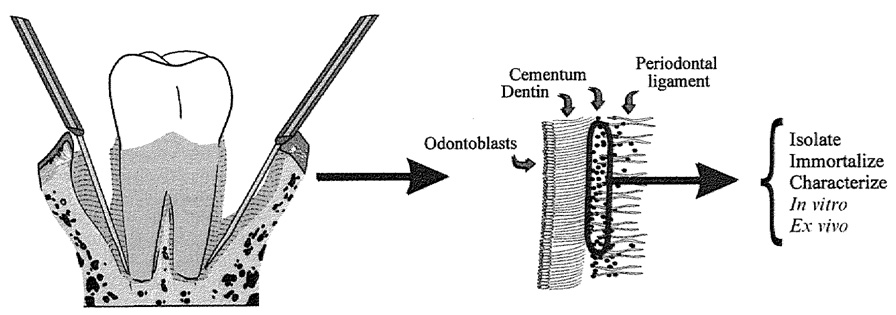
To isolate cells, molars were removed, taking care to leave tissue adherent to surrounding alveolar bone (Fig. 1). Next, molars were digested using trypsin/collagenase as described previously (6). To confirm the presence of cementoblasts,. cells cultured on chamber slides were analyzed in situ for presence of BSP and OC mRNAs.
Fig. 1.
Model demonstrating method used for obtaining cells: using a dissecting microscope first molars are removed from surrounding bone taking care to retain some tissue attached to bone.
Strategies for maintaining cementoblasts in vitro
Cultures obtained from CD-1 mice contained both PDL fibroblasts and cementoblasts, but did not survive passage. Three strategies were used to maintain cells in vitro. 1) Immortalizing cells from CD-1 mice molars with SV40 large T antigen, wild-type (9). Expression of OC and BSP transcripts by cells in vitro was confirmed, and then cells were transformed with SV40 virus as described by D’Errico et al. (7). The parent population above is referred to as SV. Cells from the follicle area of d 26 mice were similarly isolated and immortalized. The parent follicle cell population is referred to as SVF. 2) Obtaining cells from molars of day 41 immorto-mouse (H-2kb -tsA58 transgenic mice). This immorto-mouse strain harbors a temperature sensitive (ts) mutant of the SV40 large T-antigen, under control of mouse major histocompatibility complex H-2Kb class I promoter. Cells derived from these mice are conditionally immortalized from the onset of culture, thus reducing the levels of functional TAg under specific conditions (10, 11). The parent population above is referred to as IM and has been described previously (7). 3) Obtaining cells from OC-TAg mice. To obtain a parent population of cementoblasts exclusive of the surrounding PDL fibroblasts, osteocalcin promoter-driven SV40 TAg transgenic mice (OC-TAg) provided by Dr Jolene Windle were used (8, 12). The rationale here is that only those cells expressing osteocalcin, root surface cells/cementoblasts and not PDL fibroblasts. would be immortalized and therefore survive in vitro. The above parent population is referred to as OC-CM.
Cell characterization in vitro
In situ hybridization: modification of Zeller and Rogers (6, 13, 14). Northern analysis: total cellular RNA was extracted using a modified guanidine thiocyanate procedure (15). Parathyroid hormone/parathyroid hormone related protein (PTH/PTHrP) mediated CAMP assay: confluent cells were stimulated with either vehicle (4 mm HCl/0.1 % BSA) or 0.1 µm hPTH (1–34) or 0.1 µm hPTHrP (1–34) for 10 min at 37°C as described previously (16). Probes: probes used for Northern blots and in situ hybridization were: BSP=M-BSP consists of l kb of mouse cDNA in PCR 11 (17) (a gift from Dr M. Young, NIH/NIDCR); OC=400 by of mouse OC cDNA originally cloned into pSP65 cloning vector, transferred to Bluescript SK (18); OPN=MOP-3 consists of 1 kb of mouse OPN cDNA in PCR II (19) (a gift from Dr M. Young, NIH/NIDCR); Type I collagen = consists of I kb of bovine type I collagen cloned into Bluescript (a gift from Dr M. Young, NIH/NIDCR); Type XII collagen=contains 400 bp of the carboxyl region of mouse type XII collagen cDNA cloned into Bluescript (20) (a gift from Drs S. P. Oh and B. Olsen, Harvard University); Type I PTH/PTHrP receptor (PTH I R) =1.6 kb encoding the full-length rat bone receptor (21) (a gift from Dr Harold Jüppner, Harvard University, through Dr L. McCauley, University of Michigan).
Mineralization
In vitro: von Kossa stain was performed as described previously (22, 23).
Ex vivo SCID mice
Squares of Collagraft® were soaked for 30–45 min in media and then compressed between filter paper to remove air. Follicle cells from 3-d-old mice immortalized with SV40 TAg (SVF), human periodontal ligament cells (PDL cells), and cementoblasts (OC-CM), were absorbed into the prepared Collagraft and held at 37°C until implantation. SCID mice were anesthetized using methoxyfluorane, midsagittal incisions were made on the dorsa, then implants were inserted into the surgical pockets, and the sites were stapled closed. Implants were removed 39 d following surgery, fixed using 10% neutral buffered formalin, embedded in paraffin, and 5 micron sections were made and stained by H&E.
Results and discussion
In situ
As reported previously (6, 13, 24), CD-1 mice molar teeth germs, at sequential stages of development, were analyzed for localization and expression of specific proteins/genes including OPN, BSP, OC and types I and XII collagen, using immunohistochemistry and in situ hybridization. While all cells within the local region were examined for expression of the above genes, the studies presented here focus on follicle cells, periodontal ligament cells and cementoblasts. Note we define follicle cells by stage of tooth development; i.e. prior to d 31 mesenchymal cells are parallel to the surrounding bone and to the tooth germ and are defined as follicle cells. Around d 32, perpendicular orientation of fibers/cells within the newly formed ligament region is noted, and at this point we define these cells as periodontal ligament fibroblasts. Cells lining the root surface are defined as cementoblasts. As summarized in Table 1 follicle cells at early time points, d 16–26, did not express any of the proteins listed in this table, as examined by in situ hybridization or immunocytochemistry. However, Northern analysis revealed type I collagen transcripts in cells from the follicle region. Around d 26 expression of both type I and XII collagen was noted in the follicle region. Also, a pattern of selective expression for certain genes by root surface cells, cementoblasts, was emerging at this time period, e.g. d 26–31, and these genes included OPN, BSP and OC. At later stages, d 32–adult, where the PDL region was apparent, OPN protein was localized both to the PDL region and to the root surface. Also, types I and XII collagens were expressed by both PDL cells and cementoblasts. In contrast, BSP and OC remained selective to cementoblasts. This expression pattern enabled us to establish populations of PDL cells and cementoblasts, and d 41 molar roots were used due to the intense expression of BSP and OC by cementoblasts, and of type I collagen by both PDL cells and cementoblasts at this time point (Fig. 2).
Table 1.
Timed and spatial expression of proteins by follicle cells, PDL cells and cementoblasts during tooth root formation
| Probe | d 19–25 | 26–31 | 32–adult | ||
|---|---|---|---|---|---|
| DF | DF | CM | PDL | CM | |
| Type I Col | + | + | + | + | + |
| Type XII Col | − | + | −/+ | + | + |
| OPN | − | − | + | −/+ | + |
| BSP | − | − | + | − | + |
| OC | − | − | + | − | + |
DF = dental follicle; CM = cementoblast; PDL = periodontal ligament; Col = collagen; OPN = osteopontin; BSP = bone sialoprotein: OC = osteocalcin. Symbols: − no response; + response; −/+ very weak response.
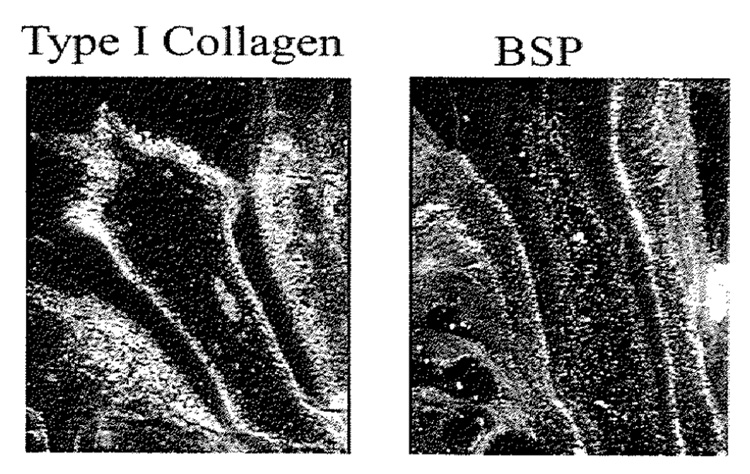
Fig. 2.
Root development, d 41. Note strong message for BSP by root surface cells, cementoblast and by osteoblasts, with absence of message by cells within the PDL region.
Cells in vitro
Subclones have been established using all 3 immortalization procedures described in the methods section (7, 8). Cementoblasts are defined as cells that express OC and/or BSP, while PDL cells are defined as cells that do not express either of these proteins. We recognize the limitation of these definitions but are optimistic that in having these cell populations available for studies “novel” genes expressed by cementoblasts, as well as others expressed by PDL cells, will be identified (see discussion below under Future directions).
Further characterization of these cells revealed that PDL cells exhibited both similar and different properties when compared with cementoblasts. Cementoblasts contained transcripts for PTH-l receptor and demonstrated an increase in cAMP upon exposure to PTHrP. The significance of PTHrP-responsiveness in cementoblast activities is not known, but studies suggest that PTHrP plays a role in promoting tooth eruption (25). Therefore, cementoblast-genes induced by PTHrP may regulate recruitment and/or activity of osteoclasts at the eruption site. Also, cementoblasts exhibited mRNA for osteoblast specific factor 2 (Osf2/ Cbfa 1), a transcription factor that binds to the osteoblast specific element 2 (OSE2 element) and that appears to be expressed by cells associated with mineralization (26, 27). OSE2 elements are present in the BSP. OC and OPN promoters. Clonal populations of PDL cells also expressed Osf2/Cbfal. This finding confirms the report of Jiang et al. (28), where they demonstrated expression of Osf2 within the developing marine tooth in association with ameloblasts, odontoblasts and PDL cells. In contrast PDL cells did not express PTH-1 receptor as measured by Northern analysis and were not responsive to PTHrP.
Mineralization properties
Cementoblasts (OC-CM) exposed to ascorbic acid and inorganic phosphate promoted mineral nodule formation within 7 d in vitro. In contrast, PDL cells and follicle cells under the same conditions did not promote mineral nodule formation. To confirm this finding under more physiological conditions SCID mice were used. As seen in Fig. 3 when cementoblasts were implanted into SCID mice subcutaneously, mineralization was noted in the implant within 6 wk. In contrast, follicle cells (Fig. 3), as well as PDL cells (data not shown) did not promote mineralization in this in vivo model.
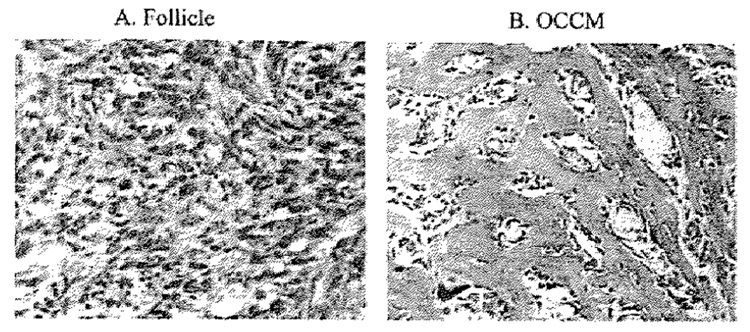
Fig. 3.
Mineralization in SCID mice: follicle cells (SVF), periodontal ligatment cells (PDL) or cementoblasts (OC-CM) were absorbed to collagraft and then implanted into SCID mice. At 6 wk implants were removed and processed for histological analysis. Cementobtasts promoted mineral formation, while follicle cells and PDL cells (data not shown) did not.
Summary and future directions
The approach used here, by first establishing genes expressed by cementoblasts. but absent from surrounding cells, follicle cells at early time points and PDL cells as the root develops in situ has enabled us to select and clone cementoblast populations separately from surrounding cell populations. Nevertheless, while there are several indicators suggesting that bone-forming cells (osteoblasts) and cementum-forming cells (cementoblasts) are different, including differences in the development of these tissues and their histological profiles, to date genes expressed specifically by cementoblasts have not been identified. Advances in molecular biology technology such as differential display. coupled with the availability of cementoblast cell lines, will enable us to explore and identify genes that differentiate the cementoblast from the osteoblast.
Furthermore, the strategy used to obtain cementoblasts in vitro is being used to develop follicle cell populations in vitro. The availability of these cells will enable us to use innovative approaches not possible in the past. such as differential display, to determine novel genes expressed by these cells when compared with osteoblasts, ameloblasts, odontoblasts and epithelial root sheath cells. Furthermore, having follicle cells in vitro should help in determining whether or not follicle cells, when appropriately triggered, have the capacity to exhibit a cementoblast phenotype.
Beyond in vitro studies these cells can be used to deliver specific genes in in vivo models of periodontal disease, thus providing information as to the optimal conditions required for promoting periodontal regeneration. Clearly, new information obtained with these advanced technologies will assist in designing more biological and hence more predictable regenerative therapies.
Acknowledgements
This work was supported by NIDCR/NIH, 1ROI DE1 3047 and 2R01 DE09532.
References
- 1.Gottlieb B. The new concept of periodontoclasia. J Periodontol. 1946;17:7–13. doi: 10.1902/jop.1946.17.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Page RC, Baab DA. A new look at the etiology and pathogenesis of early-onset periodontitis. Cementopathia revisited. J Periodontol. 1985;56:748–751. doi: 10.1902/jop.1985.56.12.748. [DOI] [PubMed] [Google Scholar]
- 3.Giannobile WV, Ryan S, Shih M-S, Su DL, Kaplan PL, Chan TCK. Recombinant human osteogenic protein-I (OP-1) stimulates periodontal wound healing in class III furcation defects. J Periodontol. 1998;69:129–137. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 4.MacNeil RL, D’Errico JA, Ouyang H, Berry J, Strayhorn C, Somerman MJ. Isolation of murine cementoblasts: unique cells or uniquely-positioned osteoblasts? Eur J Oral Sci. 1998;106 suppl 1:350–356. doi: 10.1111/j.1600-0722.1998.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 5.MacNeil RL, Sheng N, Strayhorn CL, Fisher LW, Somerman MJ. Bone sialoprotein is localized to the root surface during cementogenesis. J Bone Miner Res. 1994;9:1597–1606. doi: 10.1002/jbmr.5650091013. [DOI] [PubMed] [Google Scholar]
- 6.D’Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone. 1997;20:117–126. doi: 10.1016/s8756-3282(96)00348-1. [DOI] [PubMed] [Google Scholar]
- 7.D’Errico JA, Ouyang H, Berry JE, et al. Immortalized cementoblasts and periodontal ligament cells in culture. Bone. 1999;25:39–47. doi: 10.1016/s8756-3282(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 8.D’Errico JA, Berry JE, Ouyang H, Strayhom CL, Windle JJ, Somerman MJ. Employing a transgenic animal model to obtain cementoblasts, in vitro. J Periodoniol. doi: 10.1902/jop.2000.71.1.63. in press. [DOI] [PubMed] [Google Scholar]
- 9.Brockman WW, Christensen JB, Ryan KW, Souwaidane M, Imperiale MJ. Fate and expression of simian virus 40 DNA after introduction into marine cells under non-selective conditions. Virology. 1987;158:118–125. doi: 10.1016/0042-6822(87)90244-3. [DOI] [PubMed] [Google Scholar]
- 10.Barald KF, Lindberg KH, Hardiman K, et al. Immortalized cell lines from embryonic avian and murine otocysts: tools for molecular studies of the developing inner ear. Int J Dev Neurosci. 1997;15:523–540. doi: 10.1016/s0736-5748(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 11.Jat PS, Noble MD, Ataliotis P, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb- tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Chen H, Feng JQ, et al. Osteoblastic cell lines derived from a transgenic mouse containing the osteocalcin promoter driving SV40 T-antigen. Mol Cell Diff. 1995;3:193–212. [Google Scholar]
- 13.MacNeil RL, Berry JE, Strayhorn CL, Somerman MI. Expression of bone sialoprotein mRNA by cells lining the mouse tooth root during cementogenesis. Arch Oral Biol. 1996;41:827–835. doi: 10.1016/s0003-9969(96)00051-9. [DOI] [PubMed] [Google Scholar]
- 14.Zeller J, Rogers M. In situ hybridization and immunocyto-chemistry. In: Seidman JG, Smith JA, Struhl, editors. Current Protocols in Molecular Biology. New York, Chichester, Brisbane, Toronto, Singapore: Greene Publishing Associates and Wiley Interscience, John Wiley and Sons; 1987. pp. 14.10.11–14.16.13. [Google Scholar]
- 15.Xie WQ, Rothblum LI. Rapid, small-scale RNA isolation from tissue culture cells. Biotechniques. 1991;11:326–327. [PubMed] [Google Scholar]
- 16.Nohutcu RM, McCauley LK, Horton JE, Capen CC, Rosol TJ. Effects of hormones and cytokines on stimulation of adenylate cyclase and intracellular calcium concentration in human and canine periodontal-ligament fibroblasts. Arch Oral Biol. 1993;38:871–879. doi: 10.1016/0003-9969(93)90096-5. [DOI] [PubMed] [Google Scholar]
- 17.Young MF, Ibaraki K, Kerr JM, Lyu MS, Kozak CA. Murine bone sialoprotein (BSP): cDNA cloning, mRNA expression, and genetic mapping. Mamm Genome. 1994;5:108–111. doi: 10.1007/BF00292337. [DOI] [PubMed] [Google Scholar]
- 18.Celeste AJ, Rosen V, Bueker JL, Kriz R, Wang EA, Wozney JM. Isolation of the human gene for bone gla protein utilizing mouse and rat cDNA clones. EMBO J. 1986;5:1885–1890. doi: 10.1002/j.1460-2075.1986.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young MF, Kerr JM, Termine JD, et al. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN) Genomics. 1990;7:491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]
- 20.Oh SP, Taylor RW, Gerecke DR, Rochelle JM, Seldin MF, Olsen BR. The mouse alpha 1(XII) and human alpha 1(XII)-like collagen genes are localized on mouse chromosome 9 and human chromosome 6. Genomics. 1992;14:225–231. doi: 10.1016/s0888-7543(05)80210-1. [DOI] [PubMed] [Google Scholar]
- 21.Kong XF, Schipani E, Lanske B, et al. The rat, mouse and human genes encoding the receptor for parathyroid hormone and parathyroid hormone-related peptide are highly homologous. Biochem Biophvs Res Commun. 1994;200:1290–1299. doi: 10.1006/bbrc.1994.1591. [DOI] [PubMed] [Google Scholar]
- 22.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-El cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 23.Marsh ME, Munne AM, Vogel JJ, Cui Y, Franceschi RT. Mineralization of bone-like extracellular matrix in the absence of functional osteoblasts. J Bone Miner Res. 1995;10:1635–1643. doi: 10.1002/jbmr.5650101105. [DOI] [PubMed] [Google Scholar]
- 24.MacNeil RL, Berry JE, Strayhorn CL, Shigeyama Y, Somerman MJ. Expression of type I and XII collagen during development of the periodontal ligament in the mouse. Arch Oral Biol. 1998;43:779–787. doi: 10.1016/s0003-9969(98)00054-5. [DOI] [PubMed] [Google Scholar]
- 25.Philbrick WM, Dreyer BE, Nakchbandi IA, Karaplis AC. Parathyroid hormone-related protein is required for tooth eruption. Proc Natl Acad Sci USA. 1998;95:11846–11851. doi: 10.1073/pnas.95.20.11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfal: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 27.Somerman MJ, Berry JE, Ouyang H, et al. Defining the cementoblast: from in situ to in vitro. Am Acad Orthopaed Surg. in press. [Google Scholar]
- 28.Jiang H, Sodek J, Karsenty G, Thomas H, Ranly D, Chen J. Expression of core binding factor Osf2/Cbfa- I and bone sialo-protein in tooth development. Mech Dev. 1999;81:169–173. doi: 10.1016/s0925-4773(98)00232-9. [DOI] [PubMed] [Google Scholar]