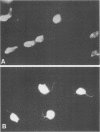
Abstract
Adherence of trichomonads to host epithelial cells appears to be a critical step in the pathogenesis of trichomoniasis. We evaluated the effect of a panel of 10 monoclonal antibodies on attachment of [35S]methionine-radiolabeled Trichomonas vaginalis strains to HeLa cell monolayers. Of 10 monoclonal antibodies, 3 totally eliminated motility of PHS2J strain trichomonads and reduced their adherence to 48 to 60% of control values (P less than 0.001). However, none of the monoclonal antibodies affected motility or adherence of STD13 strain trichomonads. Although the antibodies all reacted with PHS2J trichomonads by immunofluorescence, there was no correlation between inhibition of adherence and findings on either immunofluorescence or radioimmunoprecipitation. Direct microscopic observations showed that incubation with the monoclonal antibodies did not cause cytolysis of T. vaginalis. In quantitative cultures there was no difference in the number of colonies produced by parasites that had been incubated with antibodies that inhibited or had no effect on adherence. We conclude that our monoclonal antibodies reduced adherence not by cytotoxic effects or by competing for specific sites mediating adherence of the protozoa, but by inhibiting motility of T. vaginalis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Demes P., Gombosová A., Valent M., Yánoska A., Fabusová H., Kasmala L., Garza G. E., Metcalfe E. C. Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun. 1987 May;55(5):1037–1041. doi: 10.1128/iai.55.5.1037-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G. E. Identification and properties of Trichomonas vaginalis proteins involved in cytadherence. Infect Immun. 1988 Jan;56(1):28–33. doi: 10.1128/iai.56.1.28-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G. E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985 Dec;50(3):701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G., Smith J., Spence M. Trichomonas vaginalis: electrophoretic analysis and heterogeneity among isolates due to high-molecular-weight trichomonad proteins. Exp Parasitol. 1986 Apr;61(2):244–251. doi: 10.1016/0014-4894(86)90158-x. [DOI] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L., Metcalfe E., Garza G. E. Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun. 1986 Aug;53(2):285–293. doi: 10.1128/iai.53.2.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L. Monoclonal antibody to a major glycoprotein immunogen mediates differential complement-independent lysis of Trichomonas vaginalis. Infect Immun. 1986 Sep;53(3):697–699. doi: 10.1128/iai.53.3.697-699.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984 Apr;60(2):99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986 Apr;52(1):70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L., Smith J., Spence M. Heterogeneity of Trichomonas vaginalis and discrimination among trichomonal isolates and subpopulations with sera of patients and experimentally infected mice. Infect Immun. 1985 Sep;49(3):463–468. doi: 10.1128/iai.49.3.463-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Trichomonas vaginalis NYH286 phenotypic variation may be coordinated for a repertoire of trichomonad surface immunogens. Infect Immun. 1987 Sep;55(9):1957–1962. doi: 10.1128/iai.55.9.1957-1962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber G. E., Lemchuk-Favel L. T., Bowie W. R. Isolation of a cell-detaching factor of Trichomonas vaginalis. J Clin Microbiol. 1989 Jul;27(7):1548–1553. doi: 10.1128/jcm.27.7.1548-1553.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONIGBERG B. M. Comparative pathogenicity of Trichomonas vaginalis and Trichomonas gallinae to mice. I. Gross pathology, quantitative evaluation of virulence, and some factors affecting pathogenicity. J Parasitol. 1961 Aug;47:545–571. [PubMed] [Google Scholar]
- Heath J. P. Behaviour and pathogenicity of Trichomonas vaginalis in epithelial cell cultures: a study by light and scanning electron microscopy. Br J Vener Dis. 1981 Apr;57(2):106–117. doi: 10.1136/sti.57.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. N., Dickins C. S., Rein M. F. Use of a time-kill technique for susceptibility testing of Trichomonas vaginalis. Antimicrob Agents Chemother. 1985 Mar;27(3):332–336. doi: 10.1128/aac.27.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. N., Holmes K. K., Spence M. R., Rein M. F., McCormack W. M., Tam M. R. Geographic variation among isolates of Trichomonas vaginalis: demonstration of antigenic heterogeneity by using monoclonal antibodies and the indirect immunofluorescence technique. J Infect Dis. 1985 Nov;152(5):979–984. doi: 10.1093/infdis/152.5.979. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Poisson M. A., Rein M. F. Beta-hemolytic activity of Trichomonas vaginalis correlates with virulence. Infect Immun. 1983 Sep;41(3):1291–1295. doi: 10.1128/iai.41.3.1291-1295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. N., Ravdin J. I., Rein M. F. Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect Immun. 1985 Dec;50(3):778–786. doi: 10.1128/iai.50.3.778-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. N., Rein M. F. Zinc sensitivity of Trichomonas vaginalis: in vitro studies and clinical implications. J Infect Dis. 1982 Sep;146(3):341–345. doi: 10.1093/infdis/146.3.341. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Tam M. R., Stevens C. E., Nielsen I. O., Hale J., Kiviat N. B., Holmes K. K. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA. 1988 Feb 26;259(8):1223–1227. doi: 10.1001/jama.259.8.1223. [DOI] [PubMed] [Google Scholar]
- Krieger J. N. Urologic aspects of trichomoniasis. Invest Urol. 1981 May;18(8):411–417. [PubMed] [Google Scholar]
- Kuczyńska K., Choromański L., Honigberg B. M. Comparison of virulence of clones of two Trichomonas vaginalis strains by the subcutaneous mouse assay. Z Parasitenkd. 1984;70(2):141–146. doi: 10.1007/BF00942215. [DOI] [PubMed] [Google Scholar]
- Lushbaugh W. B., Turner A. C., Gentry G. A., Klykken P. C. Characterization of a secreted cytoactive factor from Trichomonas vaginalis. Am J Trop Med Hyg. 1989 Jul;41(1):18–28. [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. F., Sullivan J. A., Mandell G. L. Trichomonacidal activity of human polymorphonuclear neutrophils: killing by disruption and fragmentation. J Infect Dis. 1980 Oct;142(4):575–585. doi: 10.1093/infdis/142.4.575. [DOI] [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Su-Lin K. E., Honigberg B. M. Antigenic analysis of Trichomonas vaginalis strains by quantitative fluorescent antibody methods. Z Parasitenkd. 1983;69(2):161–181. doi: 10.1007/BF00926952. [DOI] [PubMed] [Google Scholar]
- Tam M. R., Stamm W. E., Handsfield H. H., Stephens R., Kuo C. C., Holmes K. K., Ditzenberger K., Krieger M., Nowinski R. C. Culture-independent diagnosis of Chlamydia trachomatis using monoclonal antibodies. N Engl J Med. 1984 May 3;310(18):1146–1150. doi: 10.1056/NEJM198405033101803. [DOI] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Barnes R. C., Kenny G. E. Antigenic heterogeneity in the 115,000 Mr major surface antigen of Trichomonas vaginalis. J Protozool. 1988 May;35(2):273–280. doi: 10.1111/j.1550-7408.1988.tb04343.x. [DOI] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Kenny G. E. A new metabolic labelling medium for Trichomonas vaginalis and Tritrichomonas foetus using 35S methionine. Proc Soc Exp Biol Med. 1986 Apr;181(4):620–623. doi: 10.3181/00379727-181-4-rc1. [DOI] [PubMed] [Google Scholar]
- Wartoń A., Honigberg B. M. Analysis of surface saccharides in Trichomonas vaginalis strains with various pathogenicity levels by fluorescein-conjugated plant lectins. Z Parasitenkd. 1983;69(2):149–159. doi: 10.1007/BF00926951. [DOI] [PubMed] [Google Scholar]