Abstract
Pancreatic carboxyl ester lipase (CEL) is in the plasma of many mammals, including humans and rats, but not mice. In vitro, CEL hydrolyzes cholesterol esters (CE) of apoB-containing lipoproteins (apoB-Lp). To study the effect of CEL on metabolism of apoB-Lp and atherosclerosis in vivo, apoE-knockout (EKO) mice, which have high plasma levels of apoB-Lp and are prone to atherosclerosis, were made to secrete CEL into plasma by introducing a transgene containing a liver-specific promoter and rat CEL cDNA. Plasma CEL activity in EKO-CEL mice was comparable to that found in rats. Evidence of modification of apoB-Lp by plasma CEL in vivo was an increase in the free cholesterol:CE ratio of apoB-Lp from mice on chow or a Western-type diet. In addition, plasma total cholesterol levels were elevated in EKO-CEL mice, with the elevation found exclusively in the apoB-Lp fraction. Associated with the increase in steady-state apoB-Lp levels was an increase in the plasma half life of very low density lipoproteins (VLDL) in EKO-CEL mice, measured by the clearance rate of injected VLDL. Interestingly, in spite of the increase of apoB-lipoproteins, the atherosclerotic lesion did not differ between EKO and EKO-CEL mice on a Western-type diet. In summary, our results demonstrate that plasma CEL modulates apoB-Lp metabolism in vivo, resulting in reduced VLDL clearance and elevated plasma cholesterol levels.
1. Introduction
Carboxyl ester lipase (CEL; EC 3.1.1.13), also called bile salt-stimulated lipase or cholesteryl ester hydrolase, is synthesized and secreted by the pancreas. It is a multiple function lipolytic enzyme (1). In vitro, it hydrolyzes cholesteryl ester (CE), retinyl ester (RE), triglycerides (TG), lysophospholipids, and ceramides, with the activity against first three substrates having the unusual dependence in vitro on the presence of millimolar concentrations of trihydroxy bile salts. Besides the pancreas, CEL is also produced by the mammary gland (2) and the liver (3-5). In addition, human macrophages and endothelial cells have been shown to synthesize and secrete CEL (6,7).
The major role of CEL was thought to be the liberation from dietary lipids of cholesterol (i.e., nonesterified or free cholesterol (FC)) and retinol from their esters by enzyme secreted by the pancreas in preparation for absorption in the intestine. Earlier studies also suggested that CEL might play a role in FC absorption (8-10) and in neonatal development and fat (TG) digestion (11). Recent studies with CEL gene-targeted mice have agreed that only CE absorption was impaired in the absence of CEL (9,12). While the absorption of FC, TG, or RE was normal in one line of CEL-null mice (12), a 40% decrease of RE absorption, however, was observed in a different line (13). Notably, the lack of CEL does not appear to affect neonatal mouse growth (12).
These results indicate that the existence of additional digestive enzyme(s) that can function in roles previously attributed to CEL. Interestingly, while CEL deficiency does not affect the total amount of cholesterol absorbed in a single meal, CEL in the intestinal lumen has been shown to influence the type of lipoprotein produced by enterocytes (14). CEL promotes the production of large chylomicrons in the intestine, which may act as a protective response to fat feeding (14).
Because it is also expressed outside of the pancreas, the functions of CEL in lipid and lipoprotein metabolism most likely extend beyond the digestive processing of lipids. CEL activity is found in the plasma of many mammals, including humans (15) and rats (16), but not mice. It is also found in the arterial vessel wall (17). In vitro, CEL modifies normal and oxidized apoB-containing lipoproteins (apoB-Lp) (17). Expression of CEL in macrophages has been shown to increase CE accumulation and promote atherosclerosis in transgenic mice (18). In addition, a recent study shows that mutations in the gene encoding CEL are associated with early-onset diabetes (19).
In the present study, we sought to investigate the effects of plasma CEL on apoB-Lp metabolism and atherosclerosis in vivo. Towards this goal, we took apoE-knock out (EKO) mice, which have high plasma levels of apoB-Lp (20) and are prone to atherosclerosis, and established EKO-CEL mice by introducing a CEL transgene containing a liver-specific promoter, thereby boosting the naturally low level of mouse plasma activity to the level that approximates that of a rat. With this model, we now show that plasma CEL modulates apoB-Lp metabolism in vivo, which results in reduced VLDL clearance and elevated plasma cholesterol levels.
2. Materials and methods
2.1. Generation and Genotyping of EKO-CEL mice
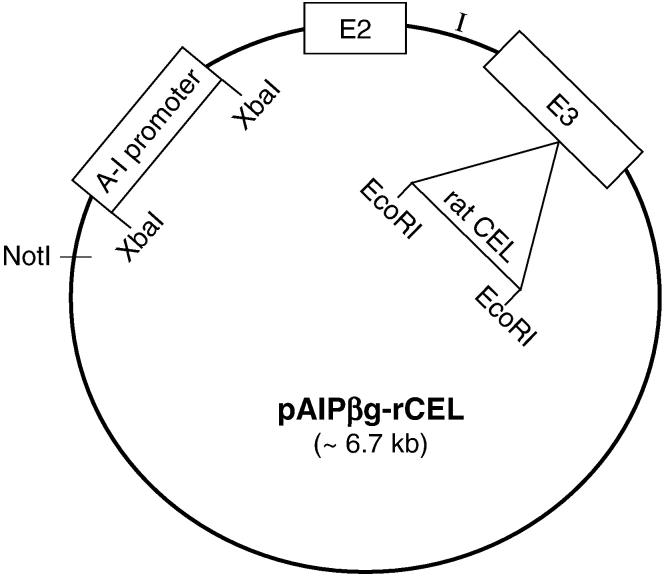
The CEL transgene was constructed in a Bluescript plasmid which had been modified previously to contain β-globin exon 2 and 3 and the intervening intron for greater expression of a transgene (21). The 324-bp hepatic-specific promoter for human apolipoprotein A-I was inserted into the plasmid multiple cloning site. The complete rat CEL cDNA (∼ 2 kb) was inserted into the EcoRI site in the exon 3 of β-globin (Fig. 1). Transgenic mice were created by microinjecting linearized plasmid construct into male pronuclei of fertilized mouse eggs as described previously (22). Tail tip DNA from mice was screened for rat CEL by PCR with primer CE1US, 5′ CTCAGTCTCTTGGGTGGTGACTCTG 3′, and primer CE9LS, 5′ GCTCAGTGCCATACCACTCTGAC 3′. Samples from transgenic mice (CEL mice) produced a 600 bp PCR product. No amplification could be detected in DNA obtained from non-transgenic mice. CEL mice were bred with EKO mice to produce EKO-CEL progeny. EKO littermates were used as controls in all experiments. Mice were fed Purina mouse chow or a Western-type diet (23) and had access to water, ad libitum.
Fig. 1. Construction of rat CEL transgene.
A Bluescript plasmid was previously modified to contain β-globin exons 2 and 3 (E2, E3) and the intervening intron (I). The 324 bp hepatic-specific promoter for apolipoprotein A-I (AI promoter) was inserted into the plasmid multiple cloning site and the complete rat CEL cDNA (∼ 2 kb) inserted in the EcoRI site of exon 3 of β-globin. The plasmid was linearized by restriction digestion with NotI and purified for microinjection.
2.2. Determination of Plasma CEL Activity
Plasma CEL activity were determined radiometrically by using cholesteryl [1-14C] oleate as the substrate, with 50 mM cholate in the reaction (24). Some plasma samples were delipidated as described previously (25) before CEL activity was determined. CEL activity was expressed in units of nmole fatty acid (FA) released/h·ml plasma.
2.3. Analysis of plasma lipids and lipoproteins
Blood samples were obtained from mice in the fasted state through retro-orbital sinus. Total plasma cholesterol (TC) and triglycerides (TG) were determined enzymatically using commercial kits (no. 126012 and no. 236691, respectively, Boehringer Mannheim Corp., Indianapolis, IN). HDL-cholesterol (HDL-C) was isolated from plasma after treatment with the HDL-reagent (Sigma, St. Louis, MO) and quantified by the same kit as for TC. Non-HDL-C (VLDL-cholesterol and LDL-cholesterol) was calculated as the difference between TC and HDL-C.
Plasma concentrations of phosphatidyl choline (PC) and lysophosphatidyl choline (lysoPC) were determined by methods modified from Switzer and Ener (26) and Shamir et al. (17). Briefly, plasma lipids were extracted with 21 volumes of chloroform/methanol (2:1, vol/vol) and washed with 4 volumes of distilled water as described by Folch et al. (27). The phospholipids were separated by thin layer chromatography (TLC) on silica gel G (Analtech Inc., Newark, DE) with two solvent systems used in the same dimension: chloroform/methanol/water, 65:35:6 and chloroform/acetone/methanol/acetic acid/water, 6:8:2:2:1. Palmitoyl PC and lysoPC (Sigma) were applied to TLC plates as standards. After visualization with iodine vapor, PC and lysoPC bands were scraped from the plates and assayed for inorganic phosphorus content according to the method of Rouser et al. (28). The ratio of lysoPC to PC was calculated for each sample.
Plasma lipoprotein total cholesterol profiles were analyzed by fast protein liquid chromatography (FPLC) using two Suprose 6 columns in series (29). Plasma samples (200 μl) pooled from mice in the same experimental group were analyzed. Lipoproteins were eluted at a constant flow rate of 0.3 ml/min with 0.15 M NaCl containing 1 mM EDTA. Total cholesterol in the eluted fractions was determined enzymatically as described above.
2.4. VLDL turnover study
The β-VLDL fraction was isolated from the plasma of EKO mice by ultracentrifugation (23). Cholesterol ester transport protein (CETP) was obtained from human lipoprotein deficient plasma (LPDP) (25). Radiolabeled VLDL was then prepared in vitro by incorporating 3H-cholesteryl oleoyl ether into β-VLDL through CETP (30). Briefly, an aliquot of β-VLDL was added to an aliquot of LPDP, which contains 40 times more total protein than the β-VLDL aliquot. Then, 1/100 volume of 2% aprotinin solution was added, followed by gradually mixing in of 50 μCi 3H-cholesteryl oleoyl ether solution in ethanol (50 μl). The mixture was capped under N2 and incubated overnight at 37°C. Radiolabeled β-VLDL was isolated from the mixture by Sephedex G50 columns. Each mouse received radiolabeled VLDL (1.6 × 106 dpm) through the femoral vein. Clearance of radiolabeled β-VLDL was determined as described previously (31). The t1/2 (half-life) of the label in the circulation was determined by checking the plasma radioactivities at different time points after injection and performing regression analysis of the data.
2.5. Quantitative Atherosclerosis Measurements
Mice were anesthetized and blood was collected via a left ventricular puncture into syringes containing ethylenediamine-tetraacetic acid (EDTA). The circulatory system was perfused with 0.9% NaCl by cardiac intraventricular canalization. The heart and ascending aorta including the aortic arch were removed, and the heart containing the aortic root was fixed in phosphate-buffered formalin and processed for the quantitative atherosclerosis assay as described previously (32).
2.6. Statistical Analysis
Comparison of different groups was performed by two-tailed Student’s t test (for normally distributed data), Mann-Whitney rank sum test (for non-normally distributed data), and repeated measures analysis of variance (ANOVA) (for VLDL turnover study). The SigmaStat software (SPSS Science, Chicago, IL) was used for statistical analyses. P < 0.05 was considered statistically significant.
3. Results
3.1. Plasma CEL activities in EKO and EKO-CEL mice
EKO-CEL mice were generated as described in Experimental Procedures. Plasma CEL activities of EKO and EKO-CEL mice were measured radiometrically by using cholesteryl [1-14C] oleate as the substrate. EKO mice (n = 11; 6 male and 5 female) had very low to undetectable plasma CEL activity (0.5 ± 0.1 nm FA/h.ml plasma), whereas EKO-CEL mice (n = 14; 7 male and 7 female) had an activity of 50.1 ± 11.8 nmole FA/h.ml plasma, which is comparable to that found in rats (30 ∼ 90 nmole FA/h.ml plasma) (16) and about four-fold higher than that in human plasma (∼ 12 nmole FA/h.ml plasma) (15,33). These relative CEL activities among different species were also confirmed by assaying CEL activities in plasma samples from wild-type mice, EKO mice, EKO-CEL mice, rats, and humans in parallel in our laboratory. To confirm that endogenous lipids were not interfering with the enzyme activity, delipidation of mouse plasma did not increase its measurable CEL activity (data not shown).
3.2. Effect of circulating CEL activity on steady-state levels of plasma lipoprotein lipids
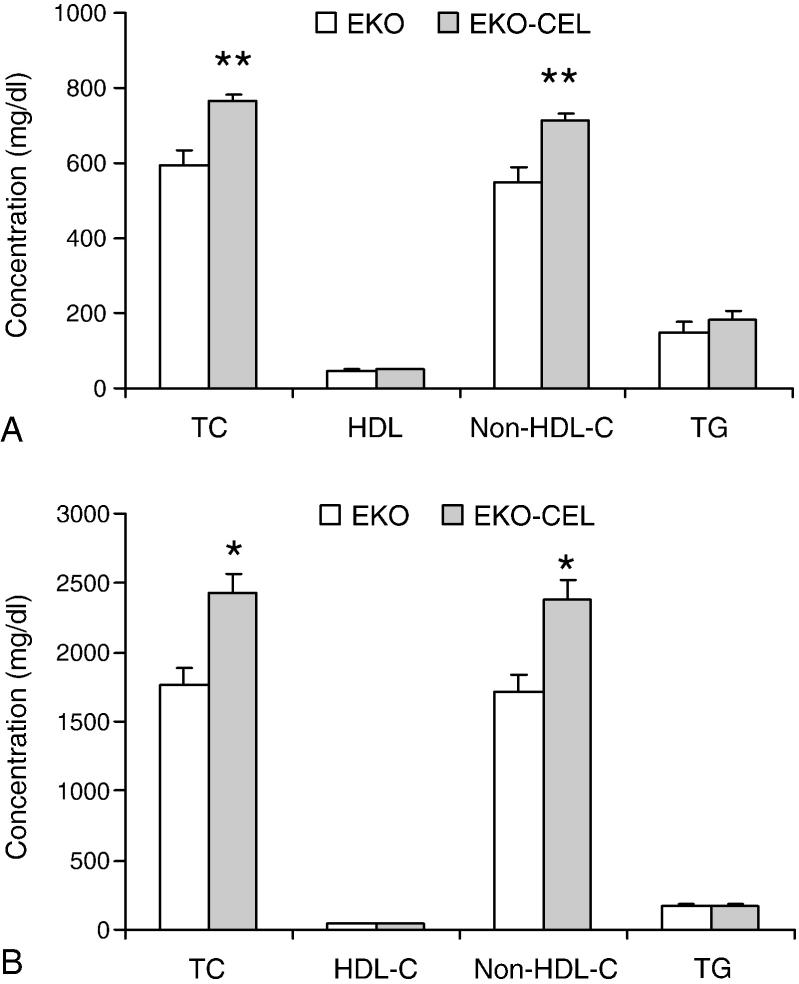
Plasma samples were collected from 2-month-old EKO mice (n=10, 4 female and 6 male) and EKO-CEL mice (n=10, 5 female and 5 male) on chow and then after 2 weeks of Western diet. Plasma total cholesterol (TC), HDL-cholesterol (HDL-C), and TG concentrations were determined enzymatically. Non-HDL-C was calculated as the difference between TC and HDL-C. Since our initial analysis did not show significant differences in plasma lipid levels between male and female mice within the same genotype, we pooled the data from male and female mice in this study. Our results showed that EKO-CEL mice had significantly higher plasma TC than EKO mice on either chow or Western diet (Fig. 2). The difference was found exclusively in the non-HDL (apoB-Lp) fraction. No significant differences between the two groups were found in the plasma TG concentrations.
Fig. 2. Plasma lipid concentrations.
Plasma samples were collected from 2 months old EKO mice (n=10) and EKO-CEL mice (n=10) on chow (A) or Western diet (B). Plasma total cholesterol (TC), HDL-cholesterol (HDL-C), and TG concentrations were determined enzymatically by using commercial kits. Non-HDL-C was calculated as the difference between TC and HDL-C. EKO-CEL mice had significantly higher plasma TC than EKO mice on either chow or Western diet. The difference was found exclusively in the non-HDL-C fraction. No significant difference between two groups was found in the plasma HDL and TG concentrations. * P < 0.05; ** P < 0.01.
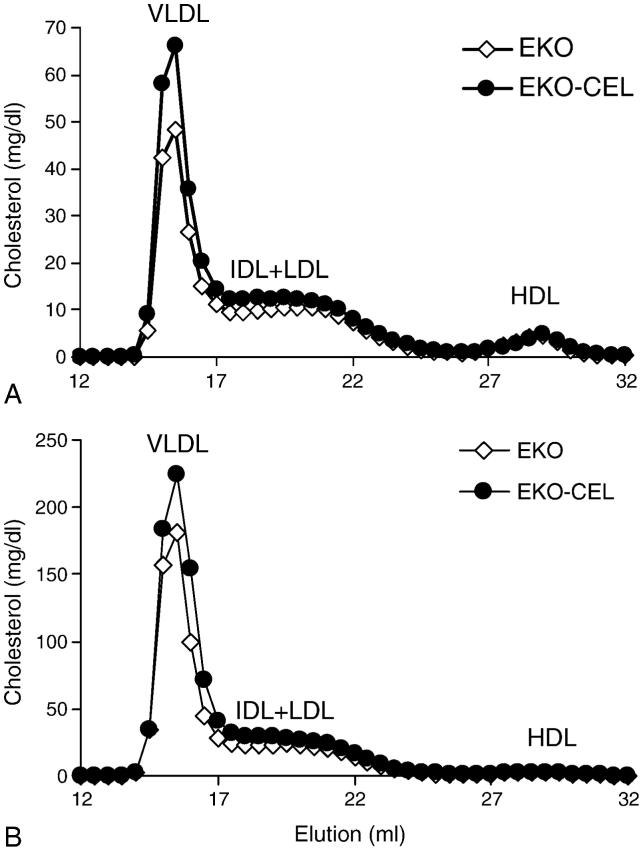
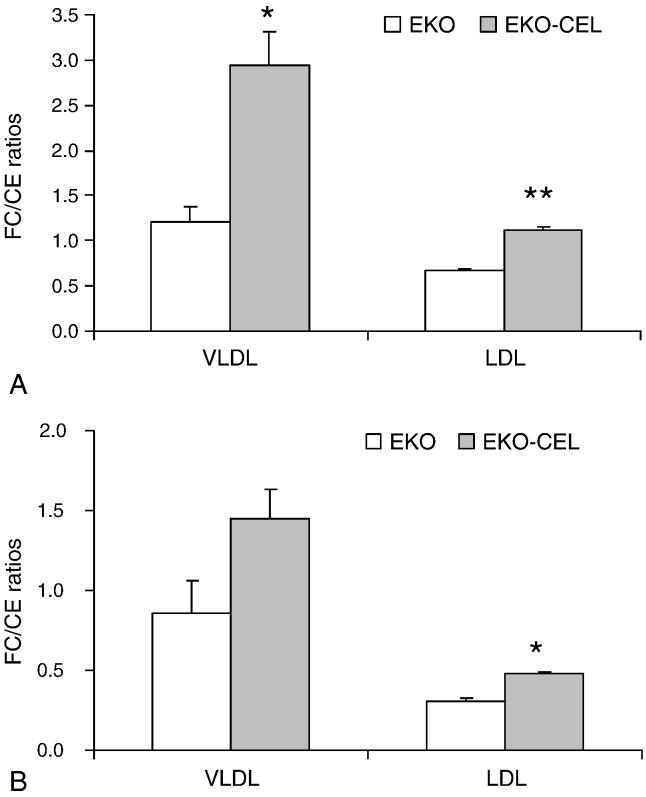
Pooled plasma samples from EKO or EKO-CEL mice, respectively, were subjected to lipoprotein cholesterol profile analysis by FPLC (Fig. 3). Consistent with the plasma cholesterol analysis above, VLDL-cholesterol from EKO-CEL mice was higher than that from EKO mice either on chow or Western diet. IDL-and LDL-cholesterol were not significantly different between EKO and EKO-CEL mice. No difference between the two groups was observed in the HDL fractions. VLDL and LDL fractions of the plasma were collected from the FPLC elution. To determine whether plasma CEL was active as a cholesteryl ester hydrolase using substrate carried by these lipoproteins, free cholesterol (FC) and CE were determined and the ratio of FC/CE calculated (Fig. 4). In both chow and Western diet-fed mice, FC to CE ratios were significantly higher in the VLDL and LDL fractions from EKO-CEL mice compared to those from EKO mice (Fig. 4). These data indicated that CEL in the plasma of EKO-CEL mice was active.
Fig. 3. Plasma lipoprotein cholesterol profiles.
Plasma samples were pooled from EKO (n=10) and EKO-CEL mice (n=10), respectively, on chow (A) or Western diet (B). FPLC was used to separate plasma lipoproteins, and the total cholesterol profiles were determined. VLDL-cholesterol from EKO-CEL mice was significantly higher than that from EKO mice either on chow or Western diet. IDL-and LDL-cholesterol also tended to be higher in EKO-CEL mice. No difference between two groups was observed in HDL fraction.
Fig. 4. Free cholesterol (FC) to cholesterol ester (CE) ratios in apoB-Lp.
VLDL and LDL fractions of the plasma were collected from the FPLC elution. FC and TC concentrations were determined enzymatically. Concentrations of CE were obtained by subtracting FC from TC. On chow diet (A), the FC/CE ratios were significantly higher in both VLDL and LDL from EKO-CEL mice compared to those from EKO mice. On Western diet (B), the FC/CE ratio in LDL was significantly higher in EKO-CEL mice and in VLDL it tended to be higher (P = 0.09) compared to that in EKO mice. * P < 0.05; ** P < 0.01.
CEL is also a lysophospholipase under some conditions. Thus, pooled plasma samples were also subjected to phospholipid analysis. LysoPC and PC concentrations in the plasma were determined by lipid extraction, separation on TLC, and colorimetric measurement of inorganic phosphorus. LysoPC to PC ratios were calculated. No significant difference was found between two groups (EKO vs. EKO-CEL: 0.65 ± 0.03 vs. 0.64 ± 0.01).
3.3. Clearance of VLDL in vivo
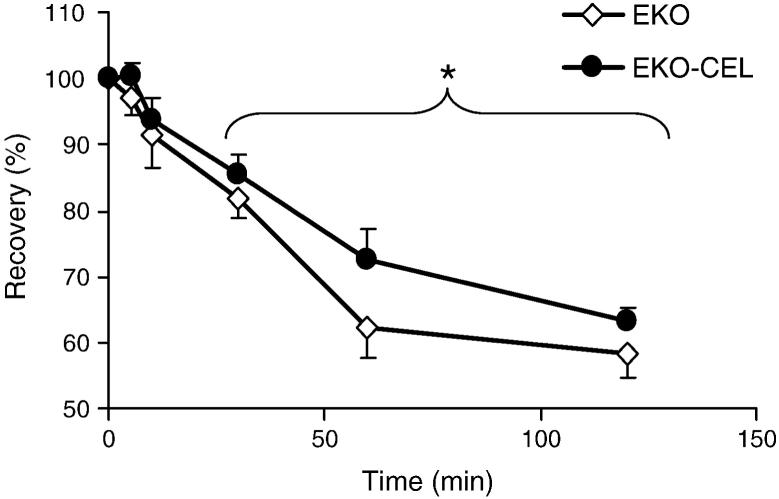
Because of the significant increase in non-HDL-C levels in EKO-CEL mice, we were interested in determining kinetically whether this was due to increased apoB-Lp production or decreased clearance. Thus, a bolus of radiolabeled VLDL was administered to chow-fed EKO and EKO-CEL mice by intravenous injection. Plasma radioactivities were determined at different time points after injection. The disappearance of radiolabeled VLDL was significantly diminished in EKO-CEL mice (n=10) compared to that in EKO mice (n=9) (Fig. 5). A regression analysis of the percentage recovery of labeled VLDL after injection showed that the plasma half life (t1/2) of VLDL was increased by about 22% in EKO-CEL vs. EKO mice (152.3 ± 9.4 min vs. 124.5 ± 14.5 min). This degree of clearance delay was roughly comparable to the % increase in the steady-state levels of non-HDL-C (Fig. 2A).
Fig. 5. Clearance of plasma VLDL on chow diet.
The turnover rate of VLDL was measured by intravenous injection of a bolus radiolabeled VLDL. Percent recovery of radioactivity at different time points after injection was determined. The rate of disappearance of radiolabeled VLDL in EKO-CEL (n=10) mice was significantly slower than that in EKO (n=9) mice 30 min after injection (P = 0.045 by repeated measures ANOVA). * P < 0.05.
3.4. Effect of circulating CEL on aortic atherosclerosis
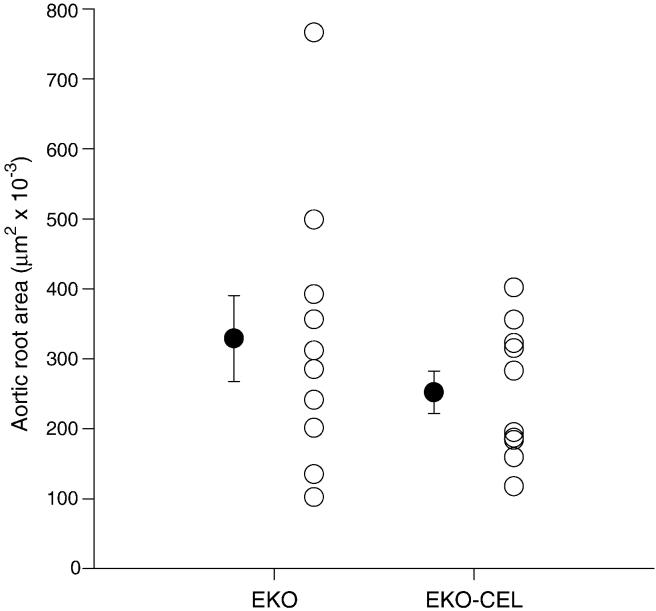
EKO and EKO-CEL mice were fed the Western diet for 10 weeks after weaning, and then subjected to aortic root lesion analysis. No significant difference in lesion size was observed between EKO (n=10; 5 male and 5 female) and EKO-CEL (n=10; 5 male and 5 female) mice (Fig. 6), with both types of mice showing relatively large lesion areas (>200,000 μm2).
Fig. 6. Atherosclerotic lesion area in the aortic root of mice on Western diet for 10 weeks.
No significant difference was observed between EKO (n=10) and EKO-CEL (n=10) mice.
4. Discussion
CEL circulates in the plasma of many mammals, including humans (13). In vitro experiments have shown that CEL modifies normal and oxidized LDL and that human aortic homogenate contains CEL activity (17), suggesting that this enzyme may influence apoB-Lp metabolism and atherosclerosis in vivo. In our mouse model, plasma CEL activity was increased by introduction of a transgene, containing rat CEL cDNA, which resulted in expression levels in plasma comparable to those in the rat and about four-fold higher than those in human. With the availability of EKO mice, which accumulate apoB-Lp in its plasma and are susceptible to atherosclerosis (23), we were able to investigate the effect of CEL on apoB-Lp metabolism and atherosclerosis in vivo by the establishment of EKO-CEL mice.
Our results show that CEL modifies apoB-Lp composition in vivo, as indicated by significant increases of FC/CE ratios in the VLDL and LDL of EKO-CEL mice either on chow or Western diet (Fig. 4). No difference was found, however, in the FC/CE ratios of HDL among different genotypes (data not shown). These results are consistent with data obtained from previous in vitro experiments. Shamir et al. (17) showed that LDL-CE was a better substrate than HDL-CE for CEL in vitro and that CEL interacted with LDL better than with HDL. In agreement with this, Caillol et al (34) reported later that CEL bound to LDL in part by specific interaction with apoB while no interaction was found with apoA-I, the principal protein of HDL. Thus, the present results in vivo are consistent with the lipoprotein interactions in vitro.
In vitro, CEL also hydrolyzes TG, and lysoPC (17). In vivo, plasma CEL appears to hydrolyze CE preferentially because no differences in the present studies were observed in the TG levels and lysoPC/PC ratios between EKO-CEL and EKO mice. Thus, although CEL is thought to function in the intestinal lumen as a lysophospholipase (13), it appears that plasma conditions do not support a significant level of this particular activity or that the substrate is not in a form that is catalytically available.
EKO-CEL mice on both types of diet had significantly higher plasma TC concentrations than EKO mice, with the differences found exclusively in the non-HDL-C fraction (Fig. 2 and Fig. 3). This result is consistent with what Brodt-Eppley et al (35) found in a human study. They reported that high plasma CEL activity correlated with plasma TC and LDL cholesterol levels in normolipidemic human subjects, suggesting elevated CEL may be contributory to increasing plasma cholesterol and LDL levels. How CEL increases apoB-Lp has not been elucidated. Our results from the VLDL-turnover studies in EKO-CEL mice indicated that an increase in the steady-state apoB-Lp levels associated with plasma CEL activity was caused by an increase in the plasma half life (t1/2) of VLDL. The average increase in the t1/2 was 22%, which kinetically accounted for the steady-state data (Fig. 2A).
The decrease of VLDL clearance in EKO-CEL mice may have been caused by several different mechanisms. Normally, remnant lipoproteins are cleared from plasma by the liver through 3 major pathways (36,37): 1) the LDL receptor pathway, 2) the LDL-receptor-related protein (LRP) pathway, and 3) the cell surface HSPG pathway. ApoE is the critical ligand responsible for remnant lipoprotein clearance (37). Lipases, such as lipoprotein lipase (LpL) and hepatic lipase (HL), may also serve as “bridging” ligands under some circumstances (38,39), primarily through interactions with the cell surface HSPG. Particularly, in the absence of apoE, the role of HL in remnant lipoprotein removal becomes evident. In EKO mice, HL deficiency causes a further increase in the accumulation of β-VLDL resulting from an impairment in HL-facilitated remnant removal (40). We speculate that in EKO-CEL mice, circulating CEL may have competed with HL for binding apoB-Lp and channeled apoB-Lp to a less efficient clearance pathway. Clearly, further studies are needed to confirm this speculation.
In addition, CEL-mediated modifications of the composition of apoB-Lp in EKO-CEL mice may also have impaired clearance. Aviram et al (41) has reported that hydrolysis of LDL-CE by a bacterial cholesterol esterase leads to a significant reduction in cellular lipoprotein binding, presumably by a change in apoB conformation because of a reduction in the core CE content. In EKO-CEL mice, the FC/CE ratios were significantly increased in the fraction of VLDL and LDL (Fig. 4) as a result of CEL activity in the plasma and/or in the liver sinusoids. Thus, the relative reduction of CE content in the VLDL of EKO-CEL mice may have contributed to decreased VLDL clearance from plasma.
Although CEL requires millimolar concentrations of bile salts for its maximal activity in vitro, it is important to note that plasma CEL was active in vivo. In plasma, the concentration of bile acid is ∼ 10 μM (42) and in postprandial portal plasma is ∼ 10-fold greater (43). The concentration of bile salts in this range is well in excess of the nanomolar levels shown to affect the conformation of CEL (44). Our data suggest that the amounts of circulating bile salts are sufficient for the activity of CEL to hydrolyze lipoprotein-associated CE. Aviram et al also found cholesterol esterase activity toward LDL in plasma and in cells of the arterial wall such as macrophages and endothelial cells, and concluded, in agreement with our results, that cholesterol esterase modification of LDL may take place in vivo (41).
Human aortic homogenate contains CEL activity (17). Consistent with this, human macrophages and endothelial cells have been shown to synthesize and secrete CEL (6,7). In vitro, CEL has also been shown to stimulate smooth muscle cell proliferation (45). These findings suggest a potential role of CEL in atherogenesis. Our present study, however, did not find significant effects of circulating CEL on aortic atherosclerosis in Western diet-fed EKO mice. Interestingly, a recent study has demonstrated that CEL expression in macrophages promotes atherosclerosis in EKO mice (18). These results are not necessarily conflicting, for a number of reasons. First, in the previous study (18), the Western-type dietary period was only for 8 weeks and the resulting lesion was much smaller than the lesion in the present study. It could be that if the atherogenic stimulus had been longer as in our study, the difference related to CEL expression would have narrowed or disappeared. Second, local (i.e., arterial wall) vs. systemic effects of CEL may not be equivalent. Indeed, CEL, like lipoprotein lipase, for example, could be envisioned to be pro- or anti-atherogenic under different circumstances. For example, inside macrophages, CEL is not likely to be a CE hydrolyzing enzyme. Indeed, CEL in macrophages was found to function as a hydrolase for ceramide and lysoPC (18). Lower levels of ceramide and lysoPC promoted the accumulation of CE and decreased cholesterol efflux in CEL-expressing macrophages, which lead to more atherosclerosis (18). In contrast, in the circulation, as we have shown in this study, CEL hydrolyzes CE and increases FC/CE ratios in atherogenic apoB-Lp (Fig. 4). This modification of apoB-Lp composition could be beneficial because a decrease of CE content in apoB-Lp is anti-atherogenic even in the presence of an elevated level of apoB-Lp (46-48) and could negate the atherogenicity of the elevated non-HDL-C levels in the EKO-CEL mice. Also, in the study of Aviram et al (41), cholesterol esterase-modified LDL was less well taken up by J774 macrophages, which could be another counter-balance to the atherogenic potential of CEL.
Yet another possibility is that the level of circulating CEL in EKO-CEL mice achieved in our study was too low to exert a putative pro-atherogenic effect; we do not favor this explanation, however, because it was comparable to that in rats (16), and in excess of that observed in humans (15,33).
In conclusion, we have shown that circulating CEL can modify apoB-Lp composition and turnover in vivo, resulting in their decreased clearance and elevated plasma cholesterol levels. These changes, however, were not sufficient to affect the development of atherosclerosis in Western diet-fed EKO mice, perhaps because the normally pro-atherogenic factors of delayed apoB-Lp clearance and increased total cholesterol levels were compensated for by less uptake of these lipoproteins in the arterial wall.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health to E.A.F. (HL58541 and DK44498) and L.L. (AG025949), and from the American Heart Association to L.L. (0455304B). We greatly appreciate intellectual input from Dr. Jan L. Breslow, human apoA-I promoter DNA from Dr. Jonathan D. Smith, and technical assistance from Douglas Chang and Joanna Halkias.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang CS, Hartsuck JA. Bile salt-activated lipase. A multiple function lipolytic enzyme. Biochim Biophys Acta. 1993;1166:1–19. doi: 10.1016/0005-2760(93)90277-g. [DOI] [PubMed] [Google Scholar]
- 2.Blackberg L, Lombardo D, Hernell O, et al. Bile salt-stimulated lipase in human milk and carboxyl ester hydrolase in pancreatic juice: are they identical enzymes? FEBS Lett. 1981;136:284–8. doi: 10.1016/0014-5793(81)80636-9. [DOI] [PubMed] [Google Scholar]
- 3.Zolfaghari R, Harrison EH, Ross AC, Fisher EA. Expression in Xenopus oocytes of rat liver mRNA coding for a bile salt-dependent cholesteryl ester hydrolase. Proc Natl Acad Sci U S A. 1989;86:6913–6. doi: 10.1073/pnas.86.18.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camulli ED, Linke MJ, Brockman HL, Hui DY. Identity of a cytosolic neutral cholesterol esterase in rat liver with the bile salt stimulated cholesterol esterase in pancreas. Biochim Biophys Acta. 1989;1005:177–82. doi: 10.1016/0005-2760(89)90184-7. [DOI] [PubMed] [Google Scholar]
- 5.Zolfaghari R, Harrison EH, Han JH, et al. Tissue and species differences in bile salt-dependent neutral cholesteryl ester hydrolase activity and gene expression. Arterioscler Thromb. 1992;12:295–301. doi: 10.1161/01.atv.12.3.295. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Hui DY. Modified low density lipoprotein enhances the secretion of bile salt-stimulated cholesterol esterase by human monocyte-macrophages. species-specific difference in macrophage cholesteryl ester hydrolase. J Biol Chem. 1997;272:28666–71. doi: 10.1074/jbc.272.45.28666. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Hui DY. Synthesis and secretion of the pancreatic-type carboxyl ester lipase by human endothelial cells. Biochem J. 1998;329(Pt 3):675–9. doi: 10.1042/bj3290675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamir R, Johnson WJ, Zolfaghari R, et al. Role of bile salt-dependent cholesteryl ester hydrolase in the uptake of micellar cholesterol by intestinal cells. Biochemistry. 1995;34:6351–8. doi: 10.1021/bi00019a013. [DOI] [PubMed] [Google Scholar]
- 9.Howles PN, Carter CP, Hui DY. Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene-targeted mice. J Biol Chem. 1996;271:7196–202. doi: 10.1074/jbc.271.12.7196. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Candales A, Grosjlos J, Sasser T, et al. Dietary induction of pancreatic cholesterol esterase: a regulatory cycle for the intestinal absorption of cholesterol. Biochem Cell Biol. 1996;74:257–64. doi: 10.1139/o96-027. [DOI] [PubMed] [Google Scholar]
- 11.Mehta NR, Jones JB, Hamosh M. Lipases in preterm human milk: ontogeny and physiologic significance. J Pediatr Gastroenterol Nutr. 1982;1:317–26. doi: 10.1097/00005176-198201030-00007. [DOI] [PubMed] [Google Scholar]
- 12.Weng W, Li L, van Bennekum AM, et al. Intestinal absorption of dietary cholesteryl ester is decreased but retinyl ester absorption is normal in carboxyl ester lipase knockout mice. Biochemistry. 1999;38:4143–9. doi: 10.1021/bi981679a. [DOI] [PubMed] [Google Scholar]
- 13.Hui DY, Howles PN. Carboxyl ester lipase: structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. J Lipid Res. 2002;43:2017–30. doi: 10.1194/jlr.r200013-jlr200. [DOI] [PubMed] [Google Scholar]
- 14.Kirby RJ, Zheng S, Tso P, et al. Bile salt-stimulated carboxyl ester lipase influences lipoprotein assembly and secretion in intestine: a process mediated via ceramide hydrolysis. J Biol Chem. 2002;277:4104–9. doi: 10.1074/jbc.M107549200. [DOI] [PubMed] [Google Scholar]
- 15.Lombardo D, Montalto G, Roudani S, et al. Is bile salt-dependent lipase concentration in serum of any help in pancreatic cancer diagnosis? Pancreas. 1993;8:581–8. doi: 10.1097/00006676-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Harrison EH. Bile salt-dependent, neutral cholesteryl ester hydrolase of rat liver: possible relationship with pancreatic cholesteryl ester hydrolase. Biochim Biophys Acta. 1988;963:28–34. doi: 10.1016/0005-2760(88)90334-7. [DOI] [PubMed] [Google Scholar]
- 17.Shamir R, Johnson WJ, Morlock-Fitzpatrick K, et al. Pancreatic carboxyl ester lipase: a circulating enzyme that modifies normal and oxidized lipoproteins in vitro. J Clin Invest. 1996;97:1696–704. doi: 10.1172/JCI118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodvawala A, Ghering AB, Davidson WS, Hui DY. Carboxyl ester lipase expression in macrophages increases cholesteryl ester accumulation and promotes atherosclerosis. J Biol Chem. 2005;280:38592–8. doi: 10.1074/jbc.M502266200. [DOI] [PubMed] [Google Scholar]
- 19.Raeder H, Johansson S, Holm PI, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006;38:54–62. doi: 10.1038/ng1708. [DOI] [PubMed] [Google Scholar]
- 20.Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr. 1995;15:495–518. doi: 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- 21.Palmiter RD, Sandgren EP, Avarbock MR, et al. Heterologous introns can enhance expression of transgenes in mice. Proc Natl Acad Sci U S A. 1991;88:478–82. doi: 10.1073/pnas.88.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh A, Ito Y, Breslow JL. High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem. 1989;264:6488–94. [PubMed] [Google Scholar]
- 23.Plump AS, Smith JD, Hayek T, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–53. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 24.Harrison EH, Smith JE, Goodman DS. Unusual properties of retinyl palmitate hydrolase activity in rat liver. J Lipid Res. 1979;20:753–9. [PubMed] [Google Scholar]
- 25.Morton RE, Steinbrunner JV. Determination of lipid transfer inhibitor protein activity in human lipoprotein-deficient plasma. Arterioscler Thromb. 1993;13:1843–51. doi: 10.1161/01.atv.13.12.1843. [DOI] [PubMed] [Google Scholar]
- 26.Switzer S, Eder HA. Transport of lysolecithin by albumin in human and rat plasma. J Lipid Res. 1965;6:506–11. [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 28.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–6. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 29.Kieft KA, Bocan TM, Krause BR. Rapid on-line determination of cholesterol distribution among plasma lipoproteins after high-performance gel filtration chromatography. J Lipid Res. 1991;32:859–66. [PubMed] [Google Scholar]
- 30.Weng W, Breslow JL. Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility. Proc Natl Acad Sci U S A. 1996;93:14788–94. doi: 10.1073/pnas.93.25.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aalto-Setala K, Bisgaier CL, Ho A, et al. Intestinal expression of human apolipoprotein A-IV in transgenic mice fails to influence dietary lipid absorption or feeding behavior. J Clin Invest. 1994;93:1776–86. doi: 10.1172/JCI117163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–11. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swan JS, Hoffman MM, Lord MK, Poechmann JL. Two forms of human milk bile-salt-stimulated lipase. Biochem J. 1992;283(Pt 1):119–22. doi: 10.1042/bj2830119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caillol N, Pasqualini E, Mas E, et al. Pancreatic bile salt-dependent lipase activity in serum of normolipidemic patients. Lipids. 1997;32:1147–53. doi: 10.1007/s11745-997-0147-4. [DOI] [PubMed] [Google Scholar]
- 35.Brodt-Eppley J, White P, Jenkins S, Hui DY. Plasma cholesterol esterase level is a determinant for an atherogenic lipoprotein profile in normolipidemic human subjects. Biochim Biophys Acta. 1995;1272:69–72. doi: 10.1016/0925-4439(95)00083-g. [DOI] [PubMed] [Google Scholar]
- 36.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–92. [PubMed] [Google Scholar]
- 37.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 38.Williams KJ, Fless GM, Petrie KA, et al. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein(a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J Biol Chem. 1992;267:13284–92. [PubMed] [Google Scholar]
- 39.Ji ZS, Dichek HL, Miranda RD, Mahley RW. Heparan sulfate proteoglycans participate in hepatic lipaseand apolipoprotein E-mediated binding and uptake of plasma lipoproteins, including high density lipoproteins. J Biol Chem. 1997;272:31285–92. doi: 10.1074/jbc.272.50.31285. [DOI] [PubMed] [Google Scholar]
- 40.Mezdour H, Jones R, Dengremont C, et al. Hepatic lipase deficiency increases plasma cholesterol but reduces susceptibility to atherosclerosis in apolipoprotein E-deficient mice. J Biol Chem. 1997;272:13570–5. doi: 10.1074/jbc.272.21.13570. [DOI] [PubMed] [Google Scholar]
- 41.Aviram M, Keidar S, Rosenblat M, Brook GJ. Reduced uptake of cholesterol esterase-modified low density lipoprotein by macrophages. J Biol Chem. 1991;266:11567–74. [PubMed] [Google Scholar]
- 42.Campbell CB, McGuffie C, Powell LW. The measurement of sulphated and non-sulphated bile acids in serum using gas-liquid chromatography. Clin Chim Acta. 1975;63:249–62. doi: 10.1016/0009-8981(75)90045-5. [DOI] [PubMed] [Google Scholar]
- 43.Angelin B, Bjorkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–31. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobson PW, Wiesenfeld PW, Gallo LL, et al. Sodium cholate-induced changes in the conformation and activity of rat pancreatic cholesterol esterase. J Biol Chem. 1990;265:515–21. [PubMed] [Google Scholar]
- 45.Auge N, Rebai O, Lepetit-Thevenin J, et al. Pancreatic bile salt-dependent lipase induces smooth muscle cells proliferation. Circulation. 2003;108:86–91. doi: 10.1161/01.CIR.0000079101.69806.47. [DOI] [PubMed] [Google Scholar]
- 46.Rudel LL, Shelness GS. Cholesterol esters and atherosclerosis-a game of ACAT and mouse. Nat Med. 2000;6:1313–4. doi: 10.1038/82110. [DOI] [PubMed] [Google Scholar]
- 47.Buhman KK, Accad M, Novak S, et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–7. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 48.Willner EL, Tow B, Buhman KK, et al. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2003;100:1262–7. doi: 10.1073/pnas.0336398100. [DOI] [PMC free article] [PubMed] [Google Scholar]