Abstract
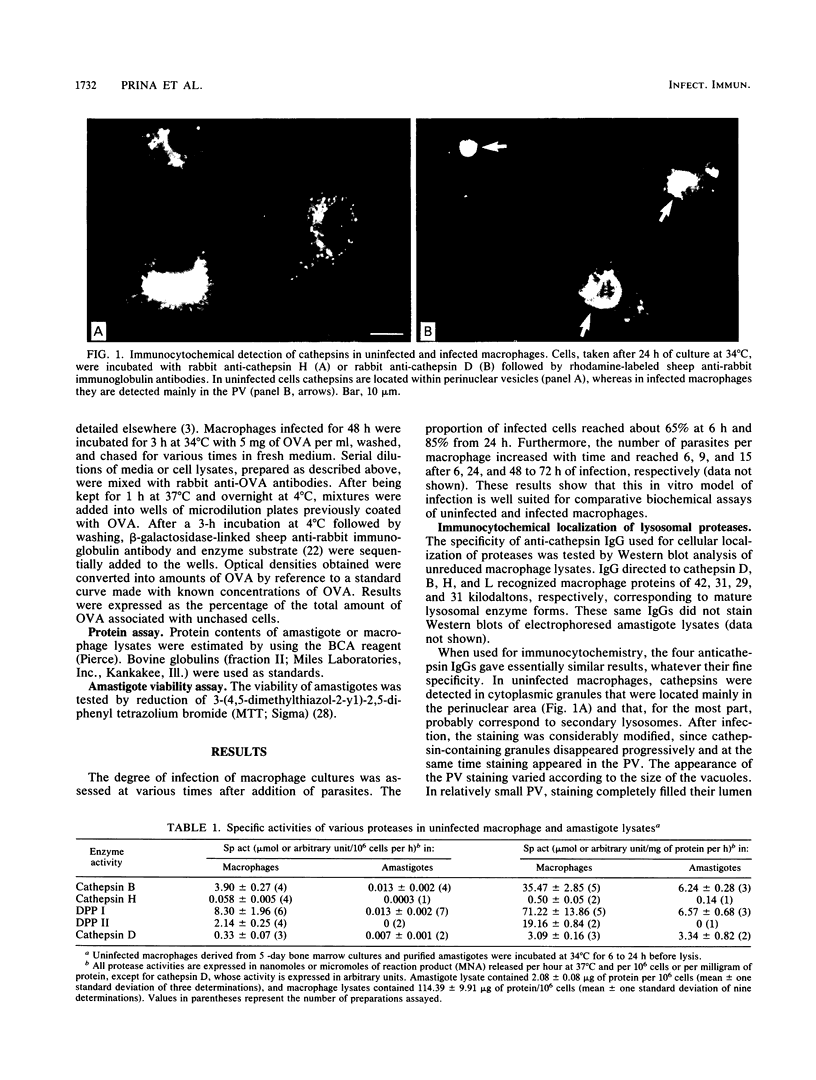
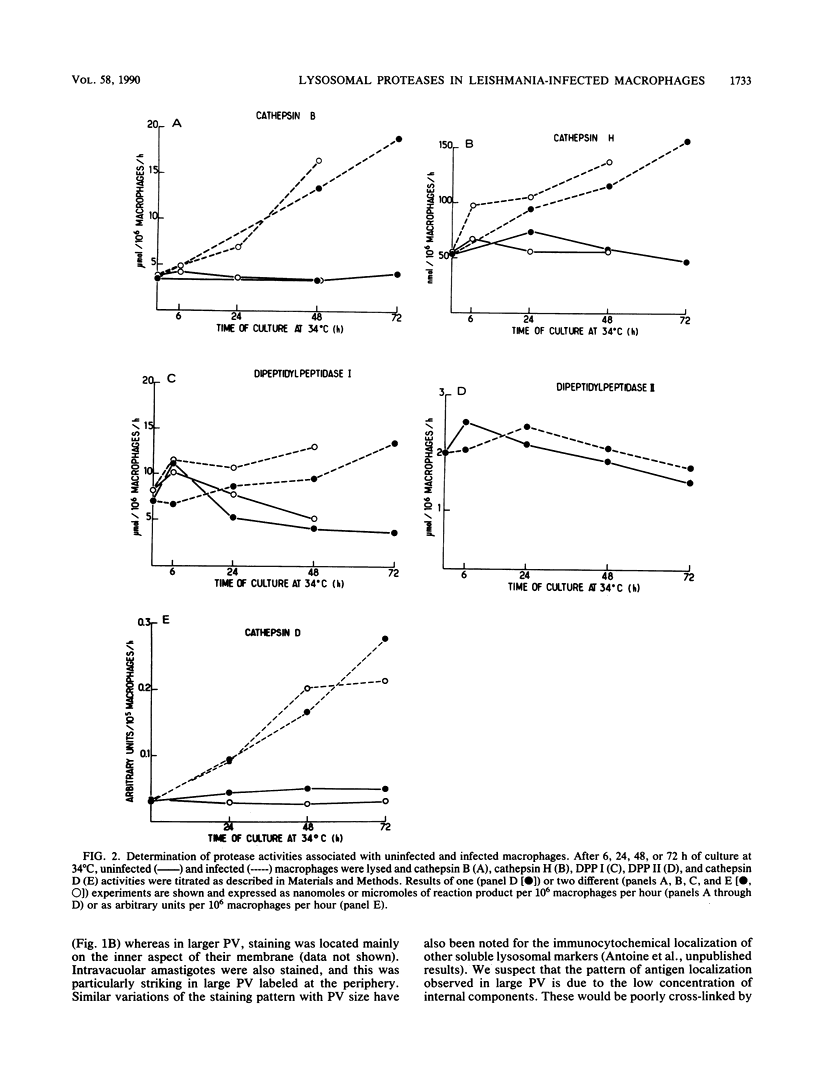
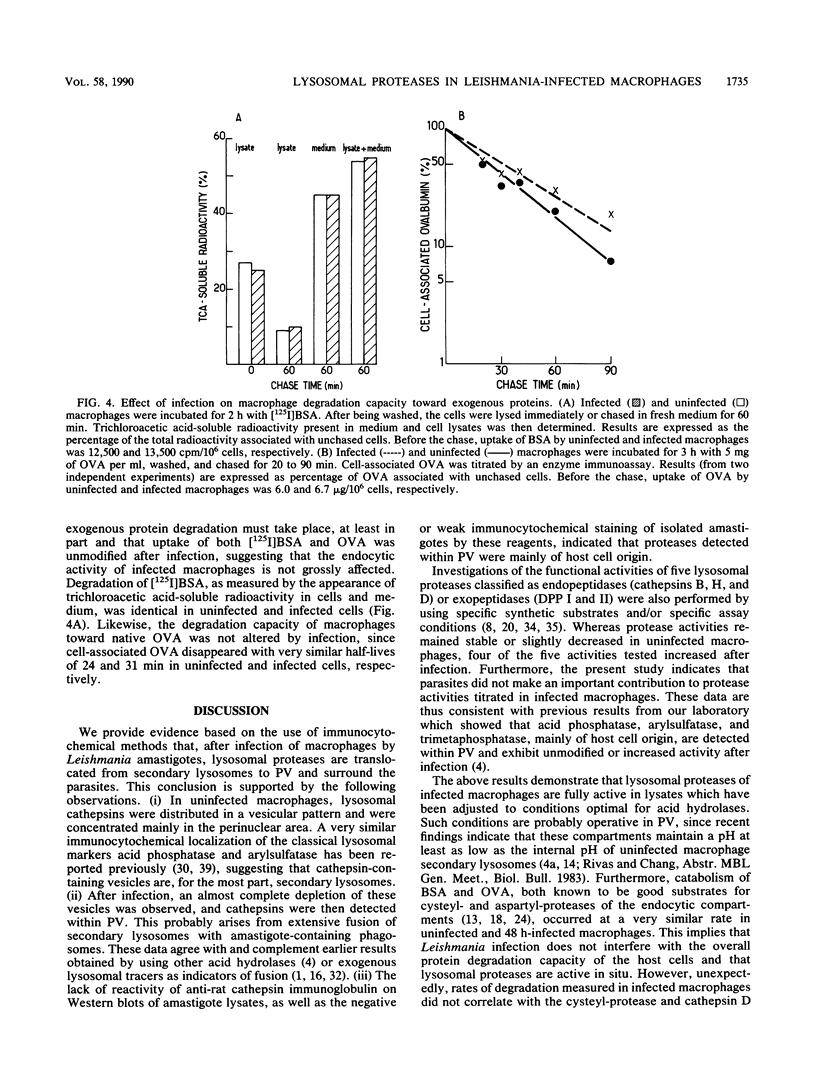
In mammalian hosts, Leishmania amastigotes are obligatory intracellular parasites of macrophages and multiply within parasitophorous vacuoles of phagolysosomal origin. To understand how they escape the harmful strategies developed by macrophages to kill ingested microorganisms, it is important to obtain information on the functional state of parasitophorous vacuole. For this purpose, we studied the intracellular distribution and activity of host lysosomal proteases in rat bone marrow-derived macrophages infected with Leishmania amazonensis amastigotes. Localization of cathepsins B, H, L, and D was investigated by using specific immunoglobulins. In uninfected macrophages, these enzymes were located in perinuclear granules (most of them were probably secondary lysosomes) which, after infection, disappeared progressively. In infected macrophages, cathepsins were detected mainly in the parasitophorous vacuoles, suggesting that the missing secondary lysosomes had fused with these organelles. Biochemical assays of various proteases (cathepsins B, H, and D and dipeptidyl peptidases I and II) showed that infection was accompanied by a progressive increase of all activities tested, except that of dipeptidyl peptidase II, which remained constant. No more than 1 to 10% of these activities could be attributed to amastigotes. These data indicate that (i) Leishmania infection is followed by an increased synthesis and/or a reduced catabolism of host lysosomal proteases, and (ii) amastigotes grow in a compartment rich in apparently fully active proteases. Unexpectedly, it was found that infected and uninfected macrophages degraded endocytosed proteins similarly. The lack of correlation in infected macrophages between increase of protease activities and catabolism of exogenous proteins could be linked to the huge increase in volume of the lysosomal compartment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Vickerman K. Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of Leishmania mexicana-infected macrophages. J Protozool. 1975 Nov;22(4):502–508. doi: 10.1111/j.1550-7408.1975.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Antoine J. C., Jouanne C., Ryter A., Benichou J. C. Leishmania amazonensis: acidic organelles in amastigotes. Exp Parasitol. 1988 Dec;67(2):287–300. doi: 10.1016/0014-4894(88)90076-8. [DOI] [PubMed] [Google Scholar]
- Antoine J. C., Jouanne C., Ryter A. Megasomes as the targets of leucine methyl ester in Leishmania amazonensis amastigotes. Parasitology. 1989 Aug;99(Pt 1):1–9. doi: 10.1017/s0031182000060960. [DOI] [PubMed] [Google Scholar]
- Antoine J. C., Jouanne C., Ryter A., Zilberfarb V. Leishmania mexicana: a cytochemical and quantitative study of lysosomal enzymes in infected rat bone marrow-derived macrophages. Exp Parasitol. 1987 Dec;64(3):485–498. doi: 10.1016/0014-4894(87)90063-4. [DOI] [PubMed] [Google Scholar]
- Antoine J. C., Prina E., Jouanne C., Bongrand P. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect Immun. 1990 Mar;58(3):779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegel D., Topper G., Rabinovitch M. Leishmania mexicana: temperature sensitivity of isolated amastigotes and of amastigotes infecting macrophages in culture. Exp Parasitol. 1983 Dec;56(3):289–297. doi: 10.1016/0014-4894(83)90074-7. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun. 1987 Mar;55(3):587–593. doi: 10.1128/iai.55.3.587-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chain B. M., Kaye P. M., Shaw M. A. The biochemistry and cell biology of antigen processing. Immunol Rev. 1988 Dec;106:33–58. doi: 10.1111/j.1600-065x.1988.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int Rev Cytol Suppl. 1983;14:267–305. [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Fong D. Cell biology of host-parasite membrane interactions in leishmaniasis. Ciba Found Symp. 1983;99:113–137. doi: 10.1002/9780470720806.ch7. [DOI] [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Eilam Y., El-On J., Spira D. T. Leishmania major: excreted factor, calcium ions, and the survival of amastigotes. Exp Parasitol. 1985 Apr;59(2):161–168. doi: 10.1016/0014-4894(85)90068-2. [DOI] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Gossrau R., Lojda Z. Study on dipeptidylpeptidase II (DPP II). Histochemistry. 1980;70(1):53–76. doi: 10.1007/BF00508846. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Thiery R., Avraméas S. Magnetic enzyme immunoassay for measuring human IgE. J Allergy Clin Immunol. 1978 Jan;61(1):23–27. doi: 10.1016/0091-6749(78)90469-4. [DOI] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Huisman W., Bouma J. M., Gruber M. Involvement of thiol enzymes in the lysosomal breakdown of native and denatured proteins. Biochim Biophys Acta. 1973 Jan 24;297(1):98–109. doi: 10.1016/0304-4165(73)90053-6. [DOI] [PubMed] [Google Scholar]
- Kahl L. P., McMahon-Pratt D. Structural and antigenic characterization of a species- and promastigote-specific Leishmania mexicana amazonensis membrane protein. J Immunol. 1987 Mar 1;138(5):1587–1595. [PubMed] [Google Scholar]
- Meshnick S. R., Eaton J. W. Leishmanial superoxide dismutase: a possible target for chemotherapy. Biochem Biophys Res Commun. 1981 Oct 15;102(3):970–976. doi: 10.1016/0006-291x(81)91633-8. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Mørland B., Mørland J. Selective induction of lysosomal enzyme activities in mouse peritoneal macrophages. J Reticuloendothel Soc. 1978 Jun;23(6):469–477. [PubMed] [Google Scholar]
- Parenti G., Willemsen R., Hoogeveen A. T., Verleun-Mooyman M., Van Dongen J. M., Galjaard H. Immunocytochemical localization of lysosomal acid phosphatase in normal and "I-cell" fibroblasts. Eur J Cell Biol. 1987 Feb;43(1):121–127. [PubMed] [Google Scholar]
- Rabinovitch M., Topper G., Cristello P., Rich A. Receptor-mediated entry of peroxidases into the parasitophorous vacuoles of macrophages infected with Leishmania Mexicana amazonensis. J Leukoc Biol. 1985 Mar;37(3):247–261. doi: 10.1002/jlb.37.3.247. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Talamas-Rohana P. Leishmania and the macrophage: a marriage of inconvenience. Immunol Today. 1989 Oct;10(10):328–333. doi: 10.1016/0167-5699(89)90188-6. [DOI] [PubMed] [Google Scholar]
- Sannes P. L., Schofield B. H., McDonald D. F. Histochemical localization of cathepsin B, dipeptidyl peptidase I, and dipeptidyl peptidase II in rat bone. J Histochem Cytochem. 1986 Aug;34(8):983–988. doi: 10.1177/34.8.3016074. [DOI] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., van Frank R. M. The use of amino acid derivatives of 4-methoxy-beta-naphthylamine for the assay and subcellular localization of tissue proteinases. Front Biol. 1975;43(4):193–249. [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Turco S. J. The lipophosphoglycan of Leishmania. Parasitol Today. 1988 Sep;4(9):255–257. doi: 10.1016/0169-4758(88)90144-5. [DOI] [PubMed] [Google Scholar]
- Waheed A., Gottschalk S., Hille A., Krentler C., Pohlmann R., Braulke T., Hauser H., Geuze H., von Figura K. Human lysosomal acid phosphatase is transported as a transmembrane protein to lysosomes in transfected baby hamster kidney cells. EMBO J. 1988 Aug;7(8):2351–2358. doi: 10.1002/j.1460-2075.1988.tb03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederanders B., Kirschke H. Antibodies to rat liver cathepsins: characterization and use for the identification of enzyme precursors. Biomed Biochim Acta. 1986;45(11-12):1421–1431. [PubMed] [Google Scholar]



