Abstract
Purpose
Commonly prescribed medications produce QT-prolongation and are associated with torsades de pointes in non-acutely ill patients. We examined patterns of QT-prolonging drug use in critically ill individuals.
Methods
An administrative critical care database was utilized to identify patients receiving drugs associated with QT-interval prolongation or torsades de pointes for ≥24 hours.
Results
Data from 212,016 individuals collected over a 63-month period was examined to identify 6,125 patients (2.9%) receiving QT-interval prolonging drugs. These individuals had a mean (±SE) age of 63.0 (±0.2) years, were predominately male (55.4%) and Caucasian (84.4%), and were exposed to QT-interval prolonging agents for a mean (±SE) 53.1 (±0.4) % of their ICU length of stay. Respiratory and cardiovascular illnesses were the most common reasons for ICU admission (17.2%, 12.0%, respectively). The most frequently administered agents were Amiodarone (23.5%), Haloperidol (19.8%), and Levofloxacin (19.7%); no other single agent accounted for more than 10% of QT-interval prolonging drugs prescribed. Coadministration of QT-prolonging drugs occurred in 1,139 patients (18.6%). These patients had higher ICU mortality rate and longer ICU lengths of stay, compared to patients not receiving coadministered drugs (p<0.001 for both). For patients receiving coadministered drugs, overlap occurred for 71.4 (±0.8) % of the time that the drugs were given. Amiodarone coadministration with antibiotics, Haloperidol coadministration with antibiotics, and Haloperidol coadministration with Amiodarone, comprised 15.2%, 13.7%, and 9.4%, of all coadministered agents, respectively.
Conclusions
QT-prolonging drugs were used in a minority of critically ill patients. Prospective evaluation in the ICU environment is necessary to determine whether administration of these agents is associated with adverse cardiac events comparable to those reported in ambulatory patients.
MeSH terms: arrhythmia, drug toxicity, database, critical care, intensive care, pharmacoepidemiology
Introduction
Many commonly prescribed medications produce QT-interval prolongation and are associated with increased risk of ventricular tachyarrhythmia, specifically torsades de pointes (1–4). Such toxicity is one of the most common causes of pharmaceutical restriction or withdrawal following regulatory approval(1;5;6). Pro-arrhythmic potential appears most pronounced when agents are prescribed in the setting of factors predisposing to arrhythmia (e.g., coronary ischemia or electrolyte abnormality), when used in conjunction with drugs impairing metabolism, or when coadministered with pharmaceuticals possessing similar QT-interval prolonging properties(1;3;5;7).
While studies examining patterns and effects of QT-interval prolonging drug use have focused on non-acutely ill patients, this phenomenon is relevant to intensivists. Many agents implicated, such as antibiotics, antiarrhythmics, and antipsychotics, are frequently prescribed in the intensive care setting(2;4;8–14). Further, polypharmacy is common practice in this environment, increasing the likelihood of untoward drug interaction(15). Finally, factors commonly encountered in the critically ill patient - pre-existing cardiac disease, shock, use of vasoconstrictive agents, organ failure, metabolic derangement, and fasting - may predispose to drug-induced arrhythmia(7). Untoward cardiac effects of QT-interval prolonging drugs might be quite pronounced in the setting of acute illness.
The purpose of this study was to profile QT-interval prolonging drug use in a large population of critically ill patients. We intend for this information to serve as a foundation for better understanding the factors that may predispose to drug induced arrhythmia in the ICU environment.
Materials and Methods
Description of Project Impact administrative database
Project Impact (Cerner Corporation, Kansas City, MO), an administrative critical care database, was the source of patient data for this study(16). This resource has been used in a number of investigations exploring many facets of ICU care(16–20). Project Impact contains descriptive information with respect to participating institutions including number of licensed hospital beds, hospital location, and whether the hospital is academically affiliated, for profit, or not for profit. Descriptive ICU information is also captured including type (medical, surgical, subspecialty), number of licensed beds, and staffing model (e.g., availability of intensivists and whether management by intensivists is mandatory). Project Impact contains anonymized clinical data including age, gender, ethnicity, payer status, and descriptions of chronic health conditions identical to that used in APACHE II scoring(21). Criteria for these conditions are as follows.
Gastrointestinal: biopsy proven cirrhosis and documented portal hypertension; episodes of prior gastrointestinal hemorrhage attributable to portal hypertension, prior episodes of hepatic failure, encephalopathy, or coma. Respiratory: Chronic restrictive, obstructive or vascular disease resulting in severe exercise restriction; documented chronic hypoxia, hypercapnia, secondary polycythemia or severe pulmonary HTN (>40mmHg); respirator dependency related to active respiratory disease such as sarcoidosis, interstitial fibrosis, TB, COPD. Cardiovascular: NY Heart Association Class IV, severe coronary artery disease, severe valvular heart disease, severe cardiomyopathy. Renal: receiving chronic renal replacement therapy; previously documented chronic renal insufficiency with the most recent serum creatinine >2.0 mg/dL. Project Impact categorizes patients as to 41 ICU admitting diagnostic categories (presented in Appendix 3). These categories are unique; a patient is assigned only one.
Appendix 3.
Distribution of patients by admitting diagnostic category
| Admitting diagnosis category | Number (%) |
|---|---|
| Cardiovascular | 738 (12.0) |
| Respiratory Infection | 531 (8.7) |
| Respiratory (not otherwise specified) | 525 (8.6) |
| Sepsis | 501 (8.2) |
| Multiple Trauma | 252 (4.1) |
| Gastrointestinal Bleeding | 248 (4.0) |
| Chronic Obstructive Pulmonary Disease | 235 (3.8) |
| Gastrointestinal (not otherwise specified) | 232 (3.8) |
| Congestive Heart Failure | 221 (3.6) |
| Heart Valve Surgery | 209 (3.4) |
| Intracerebral hemorrhage | 208 (3.4) |
| Post-Cardiac Arrest | 205 (3.3) |
| Neurologic (not otherwise specified) | 178 (3.9) |
| Coronary Artery Disease | 171 (2.8) |
| Respiratory Insufficiency after Surgery | 160 (2.6) |
| Post-Respiratory Arrest | 154 (2.5) |
| Metabolic/Renal (not otherwise specified) | 132 (2.1) |
| Gastrointestinal Perforation/Obstruction | 122 (2.0) |
| Peripheral Vascular Surgery | 109 (1.8) |
| Gastrointestinal Surgery for Neoplasm (any type) | 97 (1.6) |
| Aspiration/poisoning/toxic exposure | 96 (1.6) |
| Rhythm disturbance | 96 (1.6) |
| Craniotomy for Intracerebral hemorrhage | 74 (1.2) |
| Hemorrhagic Shock/Hypovolemia | 70 (1.1) |
| Head Trauma (confined to the head) | 68 (1.1) |
| Seizure Disorder | 61 (1.0) |
| Craniotomy for neoplasm | 59 (1.0) |
| Drug Overdose | 54 (0.9) |
| Thoracic Surgery for Neoplasm | 33 (0.5) |
| Asthma/allergy | 32 (0.5) |
| Pulmonary Edema (non-cardiogenic) | 32 (0.5) |
| Pulmonary Embolus | 32 (0.5) |
| Cardiogenic Shock | 29 (0.5) |
| Laminectomy and Other Spinal Cord Surgery | 27 (0.4) |
| Diabetic Ketoacidosis | 25 (0.4) |
| Respiratory Neoplasm | 23 (0.4) |
| Chronic Cardiovascular Disease | 23 (0.4) |
| Hypertension (hypertensive crisis) | 21 (0.3) |
| Dissecting Thoracic/Abdominal Aneurysm | 16 (0.3) |
| Renal Transplant | 15 (0.2) |
| Renal Surgery for Neoplasm | 11 (0.2) |
Identification and categorization of QT-interval prolonging medications
For the period of study, all patient records contained in our analysis file were queried to identify individuals receiving drugs associated with QT-interval prolongation and/or torsades de pointes as reported by the Center for Education and Research on Therapeutics (CERT), a publically accessible, frequently updated web-based resource(4). (Table 1) Drugs in Project Impact are assigned a unique numeric code. CERT reports a nomenclature that stratifies drugs based on risk of drug-induced arrhythmia such that a designation of '1' denotes drugs that are generally accepted to have a risk of causing torsades de pointes while '2' denotes drugs that have reported association with torsades de pointes but for which substantial evidence is lacking(4). We utilized this nomenclature for our study. We limited our analysis to patients receiving these agents (enterally or parenterally) for at least 24 hours because we felt that this exposure represented the minimal clinical risk that we felt was sufficiently important to detect. For each drug, frequency of coadministration with other QT-interval prolonging agents was determined, analogous to prior reports(2). For the purposes of this study, we defined coadministration as simultaneous administration with an overlap of at least 24 hours duration. Analysis was limited to agents comprising ≥1% of all QT-interval prolonging drugs administered.
Table 1.
Drugs associated with QT prolongation/Torsade de pointes grouped according to major class1
| Anti-infective | Antipsychotic/psychiatric | Cardiovascular | Miscellaneous | ||||
|---|---|---|---|---|---|---|---|
| Amantadine (2) | antiviral | Chlorpromazine (1) | antipsychotic | Amiodarone (1) | antiarrhythmic | Alfuzosin (2) | alpha1-antagonist |
| Azithromycin (2) | macrolide | Clozapine (2) | Bepridil (1) | CCA2 | Arsenic trioxide (1) | anti-neoplastic | |
| Clarithromycin (1) | Droperidol (1) | Disopyramide (1) | antiarrhythmic | Chloral hydrate (2) | Sedative | ||
| Erythromycin (1) | Haloperidol (1) | Dofetilide (1) | Felbamate (2) | antiepileptic | |||
| Foscarnet (2) | antiviral | Lithium (2) | mood stabilizing | Flecainide (2) | Fosphenytoin (2) | antiepileptic | |
| Gatifloxacin (2) | quinolone | Mesoridazone (1) | antipsychotic | Ibutilide (1) | Indapamide (2) | diuretic (thiazide) | |
| Gemifloxacin (2) | Paliperidone (2) | Isradipine (2) | CCA2 | Levomethadyl (1) | opiate agonist | ||
| Halofantrine (1) | anti-malarial | Pimozide (1) | Moexipril/HCTZ (2) | anti-hypertensive | Methadone (1) | opiate agonist | |
| Hydroxychloroquine (1) | anti-protazoal | Quetiapine (2) | Nicardipine (2) | CCA2 | Octreotide (2) | somatostatin analogue | |
| Levofloxacin (2) | quinolone | Risperidone (2) | Procainamide (1) | antiarrhythmic | Perflutren liquid microspheres (2) | Cardiac echo contrast agent | |
| Moxifloxacin (2) | Thioridazine (1) | Qunidine (1) | |||||
| Ofloxacin (2) | Venlafaxine (1) | antidepressant | Ranolazine (2) | Tacrolimus (2) | immunosuppressant | ||
| Pentamidine (1) | anti-protazoal | Ziprasidone (2) | antipsychotic | Sotalol (1) | Tamoxifen (2) | estrogen antagonist | |
| Sparfloxacin (1) | quinolone | Tizanidine (2) | alpha2-agonist | ||||
| Telithromycin (2) | ketolide | Anti-emetics | Salmeterol (2) | beta2-adrenergic agonist | |||
| Voriconazole (2) | triazole | Dolasetron (2) | serotonin | Sunitinib (2) | anti-neoplastic | ||
| Granisetron (2) | receptor | Vardenafil (2) | phosphodiesterase inhibitor | ||||
| Odansetron (2) | antagonist | ||||||
Numbers in parentheses correspond to likelihood that the agent is associated with torsade de pointes according to the Center for Therapeutics and Research (CERT) (4) (accessed April 2008). A ranking of '1' denotes drugs that are generally accepted to have a risk of causing torsades de pointes while '2' denotes drugs that have reported association with torsades de pointes but for which substantial evidence is lacking. Drugs not availible in the US (i.e., astemizole, cisapride, domperidone, probucol, roxythromycin, terfenadine) are excluded.
CCA - Calcium channel antagonist
Statistical analysis
Continuous data were compared using either a two sample t-test or Wilcoxon two sample test as appropriate. Categorical data were compared using either Chi Square or Fisher’s Exact Test as appropriate. Standard software was used for all computations (SYSTAT 11, Systat Software, Inc., Richmond, VA).
Human subjects protection
This study was approved by the Human Studies Committee of Washington University School of Medicine.
Results
Characteristics of hospitals and ICUs contained in dataset
We analyzed data collected from 61 hospitals over a 63-month period (January 2000 through March 2005). These hospitals had a median number of licensed beds of 489 (inter-quartile range (IQR): 342.25–636) and represented all American Hospital Association (AHA) regions with a predominance from the East North Central (24.6 %), South Atlantic (18.0 %), West North Central (13.1%), and New England (11.5%) districts. Most of these institutions were urban (54.1%) and non-academically affiliated. Only 14 university-based hospitals were represented (22.9%). (Additional detail regarding hospital characteristics is presented in Appendix 1).
Appendix 1.
Hospital Characteristics
| Characteristic | Number of hospitals (%) | Number of patients (%) |
|---|---|---|
| American Hospital Association (AHA) region | ||
| New England | 7 (11.5) | 1066 (17.4) |
| Mid-Atlantic | 4 (6.5) | 770 (12.6) |
| South Atlantic | 11 (18.0) | 1231 (20.1) |
| East North Central | 15 (24.6) | 1203 (19.6) |
| East South Central | 1 (1.6) | 61 (1.0) |
| West North Central | 8 (13.1) | 587 (9.6) |
| West South Central | 4 (6.6) | 388 (6.3) |
| Mountain | 5 (8.2) | 47 (0.8) |
| West | 4 (6.6) | 332 (5.4) |
| Not specified | 2 (3.3) | 440 (7.2) |
| Hospital classification | ||
| Urban | 33 (54.1) | 3992 (65.1) |
| Suburban | 19 (31.1) | 1313 (21.4) |
| Rural | 11 (18.0) | 881 (14.1) |
| Organizational designation | ||
| City/County | 3 (4.9) | 429 (7.0) |
| State | 1 (1.6) | 61 (1.0) |
| Community, For Profit, Non-Academic | 4 (6.6) | 53 (0.9) |
| Community, Not For Profit, Non-Academic | 42 (68.8) | 3348 (54.7) |
| Academic (University based) | 14 (22.9) | 2282 (37.2) |
The majority of the 76 ICUs sampled were mixed medical/surgical specialties (77.6%, additional information provided in Appendix 2) with a median number of licensed beds of 16 (IQR: 12.0–20.25). While a variety of ICU staffing models were reported, a minority (38.2%) mandated critical care consultation or management (i.e., the majority were not ‘closed’ ICUs).
Appendix 2.
Intensive Care Unit (ICU) specialty designations
| Designation1 | Number of ICUs (%) | Number of patients (%) |
|---|---|---|
| MICU/SICU combined | 20 (26.3) | 2363 (38.6) |
| MICU/SICU/CCU combined | 16 (21.0) | 1346 (22.0) |
| Other mixed medical, surgical/surgical subspecialty combined | 13 (17.1) | 415 (6.8) |
| MICU | 8 (10.5) | 97 (1.6) |
| Surgical/Trauma ICU | 5 (6.6) | 851 (13.9) |
| SICU | 5 (6.6) | 365 (6.0) |
| MICU/CCU combined | 3 (3.9) | 164 (2.7) |
| Trauma ICU | 3 (3.9) | 105 (1.7) |
| Cardiothoracic surgery | 2 (2.6) | 428 (7.0) |
| CCU | 1 (1.3) | 17 (0.3) |
| Other surgical/surgical subspecialty ICU combined | 2 (2.6) | 20 (0.3) |
MICU - Medical ICU; SICU -Surgical ICU; CCU -Coronary Care Unit.
Characteristics of patients receiving QT-interval prolonging drugs
Of 212,016 patients comprising the entire dataset, we identified 6,125 individuals (2.9%) receiving QT-interval prolonging drugs for duration of ≥24 hours (Table 2). While the admitting diagnostic categories of cardiovascular (12.0%), respiratory (17.2%), sepsis (8.2%), and trauma (4.1%) constituted the most common reasons for ICU admission, all admitting diagnostic categories captured by Project Impact were represented (Appendix 3). These individuals had a mean (±SE) age of 63.0 (±0.2) years, and were predominately male (55.4%) and Caucasian (84.4%). A minority of individuals had pre-existing disease, with cardiovascular (13.2%) and respiratory (10.2%) morbidity being most prevalent. Overall, patients were exposed to QT-interval prolonging agents for a mean (±SE) 53.1 (±0.4) % of their ICU length of stay. ICU readmissions accounted for 13.5% of the patients in this study.
Table 2.
Characteristics of patients receiving QT-prolonging drugs
| All patients (n=6125) | QT-prolonging drugs not coadministered (n=4986) | QT-prolonging drugs coadministered (n=1139) | p-value1 | |
|---|---|---|---|---|
| Age (years) (±SE) | 63.0 (±0.2) | 62.8 (±0.20) | 63.5 (±0.5) | 0.474 |
| Gender (% male) | 55.4 | 54.5 | 59.2 | 0.005 |
| Ethnicity (%) | 0.955 | |||
| Caucasian | 84.4 | 84.2 | 85.4 | |
| African American | 8.3 | 8.4 | 8.0 | |
| Latin/Hispanic | 4.5 | 4.6 | 4.3 | |
| Not specified/Other | 2.7 | 2.8 | 2.3 | |
| ICU Mortality Rate (%) | 11.8 | 10.9 | 16.0 | <0.001 |
| ICU LOS (days) (mean±SE) | 8.4 (±0.1) | 7.2 (±0.12) | 13.3 (±0.4) | <0.001 |
| Pre-existing disease (%) | ||||
| Cardiac | 13.2 | 13.8 | 10.5 | 0.004 |
| Respiratory | 10.2 | 10.1 | 10.7 | 0.610 |
| Renal | 3.2 | 3.3 | 2.5 | 0.195 |
| Gastrointestinal | 3.8 | 3.8 | 3.8 | 0.998 |
Refers to comparison between patients not receiving and receiving coadministered QT-prolonging drugs
Co-administration of QT-interval prolonging drugs was observed in 1139 patients (18.6% of all patients receiving QT-prolonging agents). Compared to those receiving QT-interval prolonging agents individually, co-administration was associated with a greater ICU mortality rate and longer ICU length of stay (p<0.001 for both). Similarly, compared to those receiving QT-interval prolonging agents individually, patients receiving coadministered drugs were more likely to be male, and to have lower prevalence of pre-existing cardiovascular disease (p=0.004) (Table 2).
Pattern of QT-interval prolonging drug use in critically ill patients
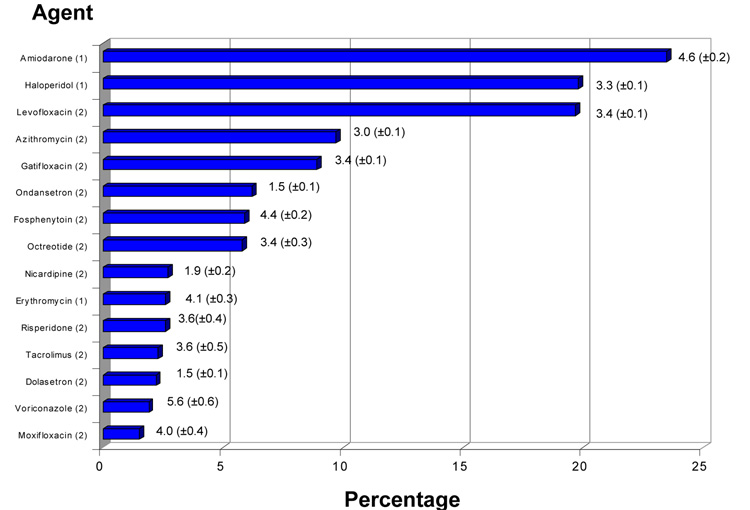
The most commonly administered QT-interval prolonging drugs were Amiodarone (accounting for 23.5% of patients receiving these agents and administered a mean (±SE) 4.6 (±0.20) days), Haloperidol (19.8%, 3.3 (±0.1) days), and Levofloxacin (19.7%, 3.4 (±0.1) days). No other single agent analyzed accounted for greater than 10% of QT-interval prolonging drugs administered in our study (Figure 1). For our analysis, drugs were classified according to reported risk of inducing QT interval prolongation and torsades de pointes. A designation of ‘1’ corresponds to a generally accepted risk of causing torsades de pointes while a designation of ‘2’ denotes reported association with torsades de pointes but for which substantial evidence is lacking(4). With the exception of amiodarone, haloperidol, and erythromycin, most drugs administered to critically ill patients in our analysis in appreciable numbers carry a ‘2’ designation.
Figure 1. Administration of QT-interval prolonging drugs in critical illness.
Frequency of QT-interval prolonging drug administration (as percentage of all QT-interval prolonging drugs administered) with associated mean (±SE) duration (days). Analysis was limited to drugs comprising at least 1% of all QT-interval prolonging drugs prescribed. Numbers in parentheses adjacent to drug names refer to the categorization with respect to risk of drug associated arrhythmia. As noted in Table 1, a designation of ‘1’ denotes generally accepted to have a risk of causing torsades de pointes while ‘2’ denotes reported association with torsades de pointes but for which substantial evidence is lacking(4). As this figure illustrates, with the exception of amiodarone, haloperidol, and erythromycin, most drugs administered to critically ill patients carry a designation of ‘2’.
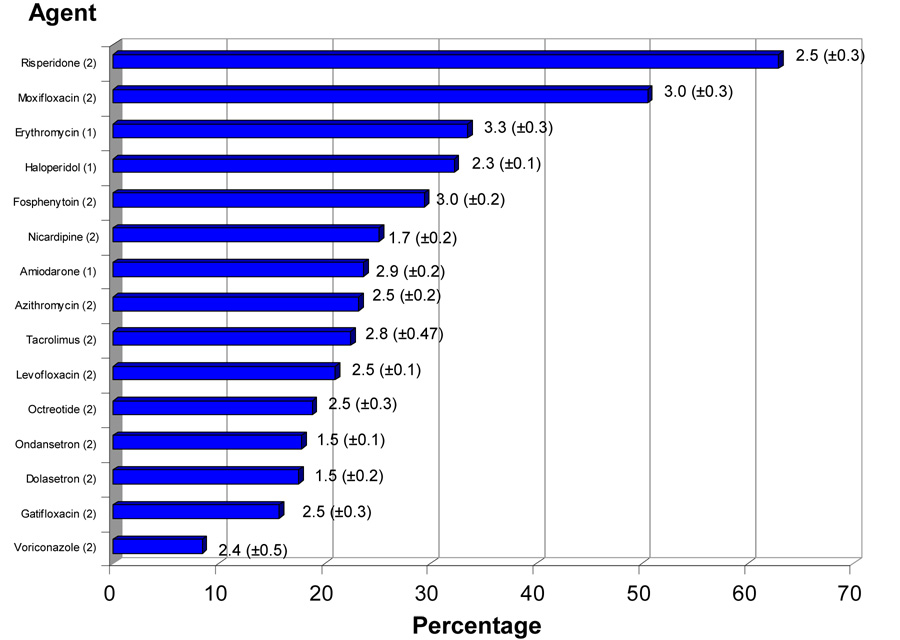
Because the effect of QT-interval prolonging drugs on risk of torsades de pointes might be potentiated when these agents are simultaneously administered with agents having comparable effect, we examined frequency and patterns of QT-interval prolonging drug coadministration. QT-interval prolonging drugs most commonly coadministered with other QT-interval prolonging agents were Respiradone (62.9% of these patients received other QT-interval prolonging agents concomitantly, mean (±SE) duration of overlap 2.48 (±0.31) days), Moxifloxacin (50.5%, 3.04 (±0.29) days) and Erythromycin (33.5%, 3.30 (±0.32) days) (Figure 2). Patterns of QT-interval prolonging drug coadministration are presented in Table 3. Amiodarone coadministration with antibiotics (i.e., levofloxacin, azithromycin, gatifloxacin, erythromycin, moxifloxacin) comprised 15.2% of all coadministered drugs (2.7 (±0.2) days), haloperidol coadministration with antibiotics comprised 13.7% of all coadministered drugs (mean duration of coadministration 2.3 (±0.1) days), and haloperidol coadministration with amiodarone comprised 9.4% of all coadministered drugs (2.7 (±0.3) days). For patients receiving coadministered QT-interval prolonging drugs, overlap occurred for 71.4 (±0.8) % of the time that the drugs were given. As noted above, both amiodarone and haloperidol carry a ‘1’ designation with respect to risk of association with torsades de pointes. Thus, the most frequent drug coadministration patterns in this population of critically patients involves at least 1 drug generally accepted as being associated with drug-induced QT-interval prolongation.
Figure 2. Frequency of Coadministration of QT-interval prolonging drugs.
Frequency of QT-interval prolonging drug coadministration is illustrated (as a percentage of all patients receiving the individual agent) with duration of coadministration (mean (±SE) days). Drug designations are as noted in Table 1 and Figure 1.
Table 3.
Frequency (%) and duration (mean±SE) (days) of most commonly coadministered QT-prolonging drugs1
 |
Percentage represents number of patients receiving specified drug combination relative to all patients receiving coadministered QT-prolonging drugs
Numbers in parentheses refer to risk of drug associated torsade de pointes as detailed in Table 1.
Discussion
Our purpose in performing this study was to profile QT-interval prolonging drug use in the ICU environment. We queried a large geographically and institutionally diverse critical care database to ascertain use of drugs associated with QT-interval prolongation and torsades de pointes. We found that approximately 3% of the individuals in our dataset received QT-interval prolonging drugs for at least 24 hours. These individuals represented a clinically heterogeneous group and were exposed to these agents on average for over one-half of their ICU lengths of stay. The majority of agents involved carry a ‘2’ designation, i.e., they have been associated with QT-prolongation and/or torsades de pointes but evidence supporting a causal link is lacking. Thus the risk posed by these agents is uncertain(4). We also examined frequency and patterns of QT-interval prolonging drug coadministration. This practice occurred in approximately 20% of individuals received QT-interval prolonging drugs. Compared to patients receiving these drugs individually, patients receiving coadministered agents had longer ICU lengths of stay and higher mortality rates, reflecting the complexity of their underlying illness. The most frequent drug coadministration patterns involved an agent with a ‘1’ designation, i.e., generally accepted as having a risk of causing torsades de pointes (4).
Important differences emerge in comparing patterns of QT-interval prolonging drug use in ambulatory and critically ill patient populations. Curtis et al found that nearly one-fourth of ambulatory patients were prescribed QT-interval prolonging drugs, most commonly clarithromycin and erythromycin(2). In contrast, in addition to identifying a much small fraction of our critically ill patient population receiving these drugs, we found that haloperidol and amiodarone were the agents most commonly prescribed. Further, it appears that coadministration of QT-interval prolonging drugs occurs approximately one-half as frequently in ambulatory individuals than in critically ill patients(2). In addition, we found that many agents commonly used in ambulatory patients (e.g., fluoxetine, sertraline, amitriptyline) are rarely prescribed in the critical care setting(2). Finally, duration of drug administration in ambulatory patients exceeded what we found for critically ill individuals. Curtis et al defined drug coadministration as overlapping prescription of at least 7 days(2). In contrast, we identified no agent in which mean duration of administration exceeded 7 days, or in which mean duration of coadministration exceeded 3 days. How differences in pattern of drug administration, particularly more brief duration of exposure, might affect risk of adverse cardiac effect in critically ill compared to non-critically ill patients is unclear.
Our study has notable limitations. While interested in understanding the relationship between QT-prolonging drug exposure and adverse cardiac events in the setting of critical illness, our analysis is entirely descriptive. Project Impact does not capture electrocardiographic data, thus precluding us from determining whether exposure to these agents was associated with increased risk of torsades de pointes, or QT interval lengthening. Further, while able to analyze drug use patterns, we did not have information as to other factors potentially influencing arrhythmia predisposition, such as organ dysfunction, electrolyte abnormality, pharmacokinetic data, or presence of congestive failure or cardiac ischemia(1;3;5;7). This and other information will be necessary to accurately gage whether QT-interval prolonging drugs pose substantial risk in acute illness(22;23). An additional limitation is that our study may underestimate QT-interval drug use in this population. We limited our analysis to individuals receiving QT-interval prolonging drugs (either alone or in combination) for no less than 24 hours because we felt that this exposure represented the minimal clinical risk that we felt was sufficiently important to detect. Nonetheless, individuals receiving briefer exposure to these agents were excluded. Finally, while Project Impact has been used by prior researchers as an investigative tool, there are inherent limitations to using administrative databases for research purposes(16;18–20). Institutions participating in Project Impact are self-selecting and may not be representative of the broad population of intensive care units(18). Likewise, while a quality assurance audit of Project Impact supports its accuracy, data fidelity is a concern of observational databases(24). Specifically, inaccurate or non-randomly distributed missing data elements may bias our findings.
The above limitations not withstanding, our findings illustrate the potential challenges of determining whether QT-prolonging drugs are associated with adverse effect in the ICU setting. Amiodarone, the most frequently administered agent in our analysis, is prescribed for arrhythmia suppression(25). Discerning whether amiodarone contributes substantially to arrhythmiagenesis in this context - and identifying safer therapeutic alternatives - may be problematic(25). Such concerns would not exist for many of the other QT-prolonging agents commonly administered to critically ill patients (antibiotics, anticonvulsants, and antipsychotics) that do not have cardiac disease as their primary indication. Nonetheless, given the small minority of patients we identified as receiving agents implicated in producing torsades de pointes, performing a sufficiently powered prospective study to demonstrate a causal effect may prove daunting(26).
Conclusion
It is becoming increasingly appreciated that pharmacoepidemiological studies are essential to detecting adverse drug effects not apparent in clinical trials conducted for purposes of regulatory approval(27–29). Such studies may acquire substantial importance in the domain of critical illness given that most pharmaceuticals haven’t been thoroughly studied in this population, that these agents are frequently used for off-label indications, and that high baseline rates of morbidity and mortality potentially obscure drug-associated toxicity(30–32). QT-interval prolonging drugs appear to be used with less frequency and shorter duration in critically ill patients relative to ambulatory individuals. However, acutely ill patients frequently receive these agents in conjunction with drugs having comparable electrophysiological effects. Whether drug toxicities are less or more pronounced in critically ill relative to non-critically ill patients is unknown. Prospective evaluation - including collection of detailed pharmokinetic and electrocardiographic data - is necessary both to determine whether use of QT-interval prolonging drugs in the ICU setting is associated with adverse cardiac events, and to understand how the risk of such events might be mitigated.
BULLET POINTS:
QT-interval prolonging drug use has not been systematically evaluated in the context of critical illness.
Relative to use reported in ambulatory populations, QT-interval prolonging drugs appear to be administered less frequently and for shorter durations in critically ill individuals.
Patients in whom QT-interval prolonging drugs are coadministered appear to be more severely ill compared to patients in whom these drugs are administered individually.
Prospective evaluation is necessary to determine whether the use of QT-interval prolonging agents in the ICU setting is associated with adverse cardiac events.
Acknowledgments
The authors wish to acknowledge the assistance and expertise of Ms. Maureen Stark and Ms. Angela Martin of the Cerner Corporation (Kansas City, MO) for preparing this data for analysis.
Footnotes
Supported in part by NIGMS GM00601
References
- 1.Roden DM. Drug-induced prolongation of the QT interval. New England Journal of Medicine. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 2.Curtis LH, Ostbye T, Sendersky V, Hutchison S, LaPointe NMA, Al-Khatib SM, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. American Journal of Medicine. 2003;114:135–141. doi: 10.1016/s0002-9343(02)01455-9. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, LaPointe NMA, Kramer JM, Califf RM. What clinicians should know about the QT interval. Journal of the American Medical Association. 2003;289:2120–2127. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 4. [accessed 16 April 2008];Arizona Center for Education and Research on Therapeutics. www.arizonacert.org.
- 5.Smalley W, Shatin D, Wysowski DK, Gurwitz J, Andrade SE, Goodman M, et al. Contraindicated use of cisapride. Impact of Food and Drug Administration Regulatory Action. Journal of the American Medical Association. 2000;284:3036–3039. doi: 10.1001/jama.284.23.3036. [DOI] [PubMed] [Google Scholar]
- 6.Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. Journal of the American Medical Association. 2002;287:2215–2220. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- 7.Zelster D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsades de points due to noncardiac drugs. Medicine. 2003;82:282–290. doi: 10.1097/01.md.0000085057.63483.9b. [DOI] [PubMed] [Google Scholar]
- 8.De Ponti F, Poluzzi E, Montanaro N. Organizing evidence on QT prolongation and occurrence of Torsades de Pointes with non-antiarrhythmic drugs: a call for consensus. European Journal of Clinical Pharmacology. 2001;57:185–209. doi: 10.1007/s002280100290. [DOI] [PubMed] [Google Scholar]
- 9.Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin and the risk of sudden death from cardiac causes. New England Journal of Medicine. 2004;351:1089–1096. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 10.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Archives of General Psychiatry. 2001;58:1161–1167. doi: 10.1001/archpsyc.58.12.1161. [DOI] [PubMed] [Google Scholar]
- 11.Straus SMJM, Bleumink GS, Dieleman JP, van der Lei J, t'Jong GW, Kingma JH, et al. Antipsychotics and risk of sudden cardiac death. Archives of Internal Medicine. 2004;164:1293–1297. doi: 10.1001/archinte.164.12.1293. [DOI] [PubMed] [Google Scholar]
- 12.Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. Thioridazine and sudden unexplained death in psychiatric in-patients. British Journal of Psychiatry. 2002;180:515–522. doi: 10.1192/bjp.180.6.515. [DOI] [PubMed] [Google Scholar]
- 13.Glassman AH, Bigger JT. Antipsychotic drugs: prolonged QT-interval, torsades de pointes, and sudden death. American Journal of Psychiatry. 2001;158:1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 14.Hennessy S, Bilker WB, Knauss JS, Margolis DJ, Kimmel SE, Reynolds RF, et al. Cardiac arrest and ventricular arrythmia in patients taking antipsychotic drugs: cohort study using administrative data. British Medical Journal. 2002;325:1070–1075. doi: 10.1136/bmj.325.7372.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman BD, McLeod HL. Challenges of implementing pharmacogenetics in the critical care environment. Nature Reviews Drug Discovery. 2004;3:88–93. doi: 10.1038/nrd1285. [DOI] [PubMed] [Google Scholar]
- 16. [accessed 20 May 2008];Cerner Corporation. ckn.cerner.com.
- 17.Freeman BD, Borecki IB, Coopersmith CM, Buchman TB. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Critical Care Medicine. 2005. 2005;33:2513–2520. doi: 10.1097/01.ccm.0000186369.91799.44. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport J, Teres D, Steingrub J, Higgins T, McGee W, Lemeshow S. Patient characteristics and ICU organizational factors that influence frequency of pulmonary artery catheterization. Journal of the American Medical Association. 2000;283:2557–2567. doi: 10.1001/jama.283.19.2559. [DOI] [PubMed] [Google Scholar]
- 19.Glance LG, Osler TM, Dick AW. Identifying quality outliers in a large, multiple institution database by using customized version of the Simplified Acute Physiology Score II and the Mortality Probability Model II. Critical Care Medicine. 2002;30:1995–2002. doi: 10.1097/00003246-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Higgins TL, McGee WT, Steingrub JS, Rapoport J, Lemeshow S, Teres D. Early predictors of prolonged intensive care unit stay: Impact of illness severity, physician staffing, and pre-intensive care unit length of stay. Critical Care Medicine. 2003;31:45–51. doi: 10.1097/00003246-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Medicine. 1985;13:818–828. [PubMed] [Google Scholar]
- 22.Sesti F, Abbott GW, Wei J, Murray KT, Saksena S, Schwartz PJ, et al. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proceedings of the National Academy of Sciences. 2000;97:10613–10618. doi: 10.1073/pnas.180223197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Splwaski I, Timothy KW, Tateyama M, Clancy CE, Malhotra A, Beggs AH, et al. Variant of SCN5A Sodium Channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 24.Cook SF, Visscher WA, Hobbs CL, Williams RL. Projct IMPACT: results from a pilot validity study of a new observational database. Critical Care Medicine. 2002;30:2765–2770. doi: 10.1097/00003246-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Roden DM. Antiarrhythmic Drugs. In: Hardman JG, Limbird LL, editors. The pharmacological basis of therapeutics. 10 ed. New York: McGraw-Hill; 2001. pp. 933–970. [Google Scholar]
- 26.Avorn J. In defense of pharmacoepidemiology - embracing the Yin and Yang of drug research. New England Journal of Medicine. 357:2219–2221. doi: 10.1056/NEJMp0706892. 20087. [DOI] [PubMed] [Google Scholar]
- 27.Psaty BM, Burke SP. Protecting the health of the public - Institute of medicine recommendations on drug safety. New England Journal of Medicine. 2007;355:1753–1755. doi: 10.1056/NEJMp068228. [DOI] [PubMed] [Google Scholar]
- 28.Roden DM. An under-recognized challenge in evaluating post-marketing drug safety. Circulation. 2005;111:246–248. doi: 10.1161/01.CIR.0000154578.45378.8C. [DOI] [PubMed] [Google Scholar]
- 29.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. New England Journal of Medicine. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 30.Freeman BD, Danner RL, Banks S, Natanson C. Safeguarding patients in clinical trials with high mortality rates. American Journal of Respiratory and Critical Care Medicine. 2001;164:190–192. doi: 10.1164/ajrccm.164.2.2011028. [DOI] [PubMed] [Google Scholar]
- 31.Palazzo M, Soni N. Critical care studies: redefining the rules. Lancet. 1999;352:1306–1307. doi: 10.1016/S0140-6736(98)02342-3. [DOI] [PubMed] [Google Scholar]
- 32.Stafford RS. Regulating off-label drug use - rethinking the role of the FDA. New England Journal of Medicine. 2008;358:1427–1429. doi: 10.1056/NEJMp0802107. [DOI] [PubMed] [Google Scholar]