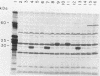
Abstract
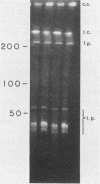
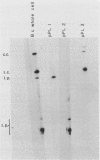
Three recombinant plasmids containing DNA from Borrelia coriaceae, the putative agent of epizootic bovine abortion, expressed antigens in Escherichia coli that reacted with antibodies specific for B. coriaceae. Two of the recombinants each expressed a single high-molecular-weight antigen. The third recombinant expressed three smaller antigens. The DNA inserts were sized and mapped. Hybridization of the cloned inserts to pulsed-field electrophoresis samples of B. coriaceae whole-cell DNA revealed the origin of two of the inserts to be located in linear plasmids. One of these, expressing three antigens, was located in a 210-kilobase linear plasmid. A second recombinant expressed a single antigen but hybridized to at least three distinct linear plasmids. The third clone also expressed a single antigen but was demonstrated to be chromosomal in origin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Coates S. R., Sheridan P. J., Hansen D. S., Laird W. J., Erlich H. A. Serospecificity of a cloned protease-resistant Treponema pallidum--specific antigen expressed in Escherichia coli. J Clin Microbiol. 1986 Mar;23(3):460–464. doi: 10.1128/jcm.23.3.460-464.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Walfield A. M., Cunningham T. M., Radolf J. D., Miller J. N., Lovett M. A. Purification and characterization of a cloned protease-resistant Treponema pallidum-specific antigen. Infect Immun. 1984 Nov;46(2):598–607. doi: 10.1128/iai.46.2.598-607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesdon J. L., Bouges Bocquet B., Débarbouillé M., Hofnung M. In situ enzyme immunodetection of surface or intracellular bacterial antigens using nitrocellulose sheets. J Immunol Methods. 1985 Nov 28;84(1-2):53–63. doi: 10.1016/0022-1759(85)90414-4. [DOI] [PubMed] [Google Scholar]
- Hayes L. J., Wright D. J., Archard L. C. Segmented arrangement of Borrelia duttonii DNA and location of variant surface antigen genes. J Gen Microbiol. 1988 Jul;134(7):1785–1793. doi: 10.1099/00221287-134-7-1785. [DOI] [PubMed] [Google Scholar]
- Howe T. R., LaQuier F. W., Barbour A. G. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect Immun. 1986 Oct;54(1):207–212. doi: 10.1128/iai.54.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., Mayer L. W., Barbour A. G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985 Feb 8;227(4687):645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- KENNEDY P. C., OLANDER H. J., HOWARTH J. A. Pathology of epizootic bovine abortion. Cornell Vet. 1960 Oct;50:417–429. [PubMed] [Google Scholar]
- Kehl K. S., Farmer S. G., Komorowski R. A., Knox K. K. Antigenic variation among Borrelia spp. in relapsing fever. Infect Immun. 1986 Dec;54(3):899–902. doi: 10.1128/iai.54.3.899-902.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Burgdorfer W., Hayes S. F., Barbour A. G. Isolation of a spirochete from the soft tick, Ornithodoros coriaceus: a possible agent of epizootic bovine abortion. Science. 1985 Oct 4;230(4721):85–87. doi: 10.1126/science.3898367. [DOI] [PubMed] [Google Scholar]
- LeFebvre R. B., Perng G. C. Genetic and antigenic characterization of Borrelia coriaceae, putative agent of epizootic bovine abortion. J Clin Microbiol. 1989 Mar;27(3):389–393. doi: 10.1128/jcm.27.3.389-393.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. T., Simon M. I., Barbour A. G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985 Jun;41(2):403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., Osburn B. I., Spezialetti R., Bushnell R. B., Stott J. L. Histopathologic changes in bovine fetuses after repeated reintroduction of a spirochete-like agent into pregnant heifers: association with epizootic bovine abortion. Am J Vet Res. 1987 Apr;48(4):627–633. [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schmidtmann E. T., Bushnell R. B., Loomis E. C., Oliver M. N., Theis J. H. Experimental and epizootiologic evidence associating Ornithodoros coriaceus Koch (Acari: Argasidae) with the exposure of cattle to epizootic bovine abortion in California. J Med Entomol. 1976 Dec 8;13(3):292–299. doi: 10.1093/jmedent/13.3.292. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Kerner T. C., Jr, Bankaitis V. A., Bassford P. J., Jr Identification and preliminary characterization of Treponema pallidum protein antigens expressed in Escherichia coli. Infect Immun. 1983 Aug;41(2):709–721. doi: 10.1128/iai.41.2.709-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J. D., van der Donk H. J., van Eijk R. V., van der Heide H. G., de Jong J. A., van Olderen M. F., Osterhaus A. B., Schouls L. M. Molecular cloning and expression of Treponema pallidum DNA in Escherichia coli K-12. Infect Immun. 1983 Oct;42(1):187–196. doi: 10.1128/iai.42.1.187-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]