Abstract
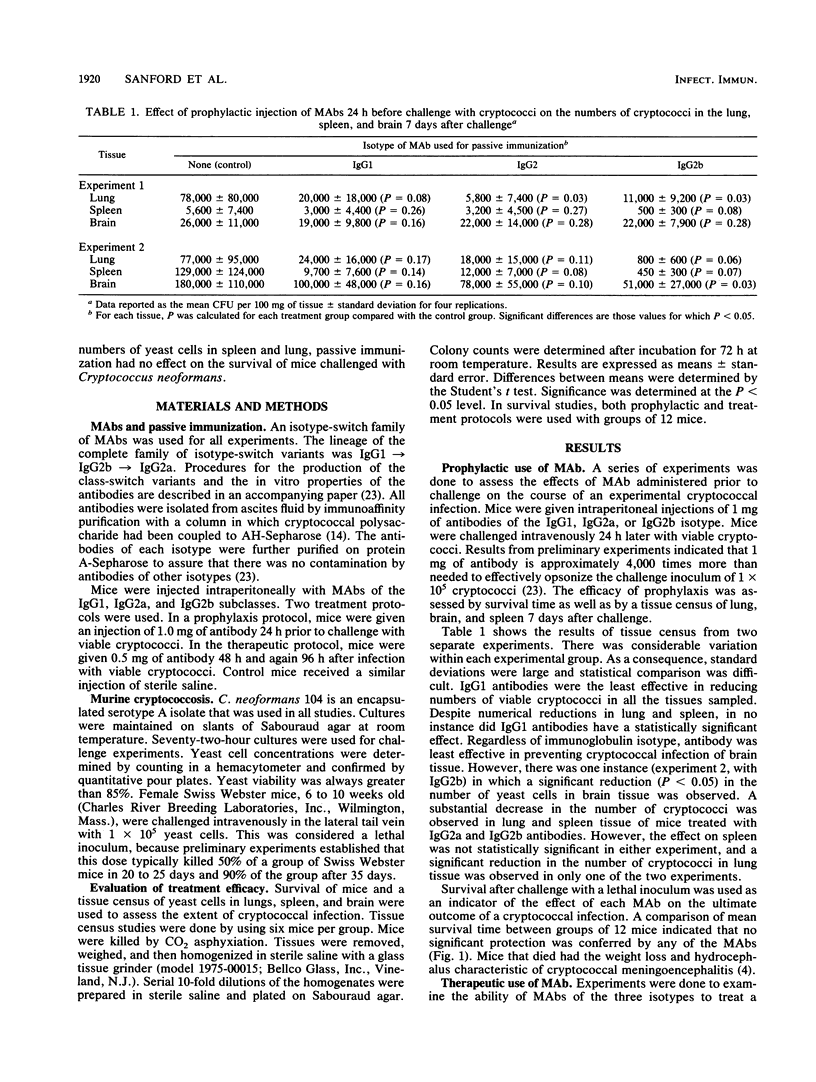
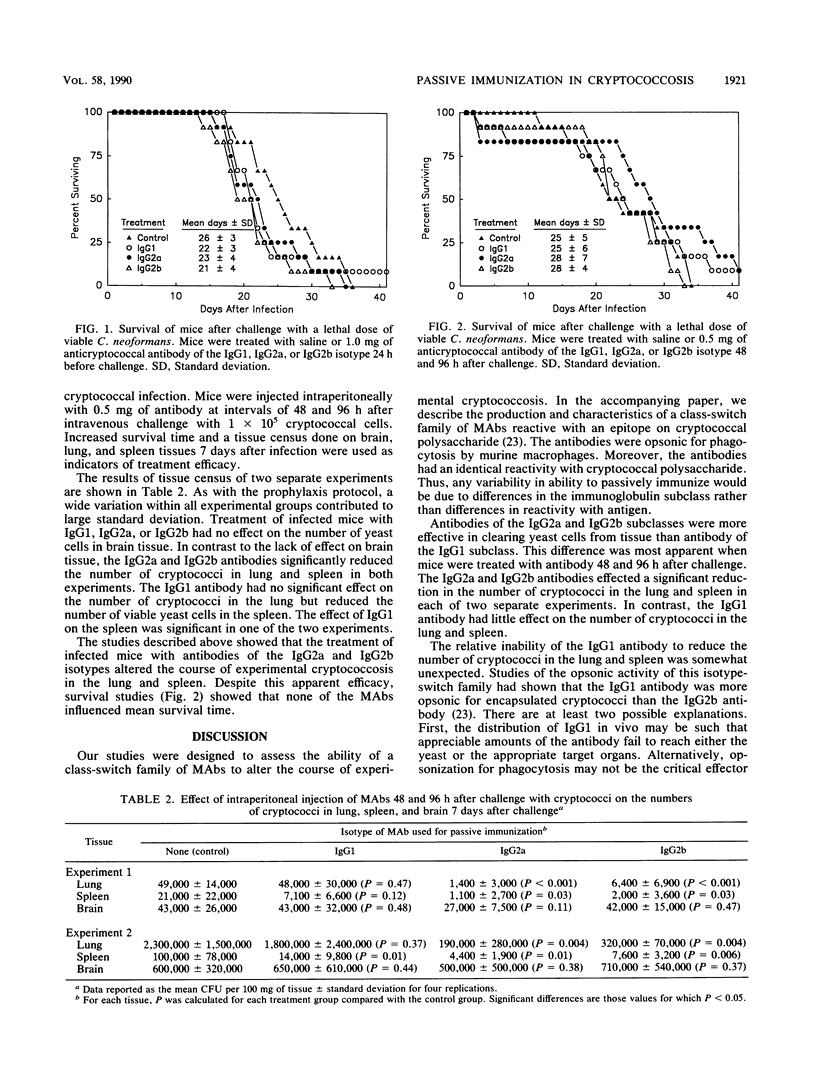
The in vivo properties of an immunoglobulin isotype-switch family of monoclonal antibodies specific for the polysaccharide capsule of Cryptococcus neoformans were examined in a murine model of cryptococcosis. Subclass-switch variants were isolated by sequential sublining of an immunoglobulin G subclass 1 (IgG1)-secreting cell line. Antibodies of the IgG1, IgG2a, and IgG2b isotypes with identical reactivities with cryptococcal polysaccharide were prepared. The antibodies had the distinct biological properties associated with the heavy chains of each respective isotype. The antibodies were used prophylactically or therapeutically in an attempt to alter the course of cryptococcal infection in mice. Survival of mice and a tissue census of the numbers of viable cryptococci in the lung, spleen, and brain were used as indicators of efficacy. Passive immunization with the IgG2a and IgG2b antibodies effected a reduction in the numbers of cryptococci in lung and spleen. Passive immunization with the IgG1 antibody was markedly less effective. Passive immunization had little or no effect on the numbers of cryptococci in brain tissue, regardless of the immunoglobulin isotype. Despite apparent efficacy with regard to reduction in the numbers of yeast cells in the lung and spleen, the results showed no improvement in survival from murine cryptococcosis. Our results indicate that passive immunization produces a modest effect on the course of murine cryptococcosis in tissues other than brain. However, under the experimental conditions used, such treatment does not have a measurable impact on the ultimate outcome of the infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur B. R., Larose Y., Tsang P., Hamel J., Ashton F., Ryan A. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect Immun. 1985 Nov;50(2):510–516. doi: 10.1128/iai.50.2.510-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Charreire J., Contrepois A., Carbon C., Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987 Mar;55(3):749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Perronne C., Barge J., Vilde J. L., Yeni P. Role of IgG and complement component C5 in the initial course of experimental cryptococcosis. Clin Exp Immunol. 1989 Dec;78(3):412–417. [PMC free article] [PubMed] [Google Scholar]
- GADEBUSCH H. H. Passive immunization against Cryptococcus neoformans. Proc Soc Exp Biol Med. 1958 Jul;98(3):611–614. doi: 10.3181/00379727-98-24123. [DOI] [PubMed] [Google Scholar]
- GORDON M. A., LAPA E. SERUM PROTEIN ENHANCEMENT OF ANTIBIOTIC THERAPY IN CRYPTOCOCCOSIS. J Infect Dis. 1964 Oct;114:373–377. doi: 10.1093/infdis/114.4.373. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis. 1982 Aug;146(2):249–254. doi: 10.1093/infdis/146.2.249. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Lee D., Insel R. A., Scheld W. M. IgG penetration into the cerebrospinal fluid in a rabbit model of meningitis. J Infect Dis. 1987 Aug;156(2):394–398. doi: 10.1093/infdis/156.2.394. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–922. [PubMed] [Google Scholar]
- Graybill J. R., Hague M., Drutz D. J. Passive immunization in murine cryptococcosis. Sabouraudia. 1981 Dec;19(4):237–244. doi: 10.1080/00362178185380411. [DOI] [PubMed] [Google Scholar]
- Horta M. F., Ramalho-Pinto F. J. Subclasses of rat IgG active in the killing of schistosomula of Schistosoma mansoni in vitro and in vivo. J Immunol. 1984 Dec;133(6):3326–3332. [PubMed] [Google Scholar]
- Kaminski M. S., Kitamura K., Maloney D. G., Campbell M. J., Levy R. Importance of antibody isotype in monoclonal anti-idiotype therapy of a murine B cell lymphoma. A study of hybridoma class switch variants. J Immunol. 1986 Feb 1;136(3):1123–1130. [PubMed] [Google Scholar]
- Kipps T. J., Parham P., Punt J., Herzenberg L. A. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med. 1985 Jan 1;161(1):1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Hermerath C. A. Benzoquinone activation of Cryptococcus neoformans capsular polysaccharide for construction of an immunoaffinity column. J Immunol Methods. 1988 Feb 24;107(1):53–58. doi: 10.1016/0022-1759(88)90008-7. [DOI] [PubMed] [Google Scholar]
- Louria D. B., Kaminski T. Passively-acquired immunity in experimental cryptococcosis. Sabouraudia. 1965 Jun;4(2):80–84. doi: 10.1080/00362176685190211. [DOI] [PubMed] [Google Scholar]
- Mujoo K., Kipps T. J., Yang H. M., Cheresh D. A., Wargalla U., Sander D. J., Reisfeld R. A. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989 Jun 1;49(11):2857–2861. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect Immun. 1986 Feb;51(2):556–562. doi: 10.1128/iai.51.2.556-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Vuong T. M., Hardy R., Reidler J., Dangle J., Herzenberg L. A., Stryer L. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984 Jan 12;307(5947):136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- Pelkonen S., Pluschke G. Use of hybridoma immunoglobulin switch variants in the analysis of the protective properties of anti-lipopolysaccharide antibodies in Escherichia coli K1 infection. Immunology. 1989 Oct;68(2):260–264. [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Lang S. D., Durack D. T. Influence of agglutinating antibody in experimental cryptococcal meningitis. Br J Exp Pathol. 1981 Dec;62(6):595–599. [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C. Contribution of complement component C5 to the pathogenesis of experimental murine cryptococcosis. Sabouraudia. 1985 Jun;23(3):225–234. doi: 10.1080/00362178585380331. [DOI] [PubMed] [Google Scholar]
- Schlageter A. M., Kozel T. R. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect Immun. 1990 Jun;58(6):1914–1918. doi: 10.1128/iai.58.6.1914-1918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka A. O., Pincus S. H., Rote N. S., Hill H. R. Protective efficacy of hybridoma type-specific antibody against experimental infection with group-B Streptococcus. J Infect Dis. 1984 Mar;149(3):363–372. doi: 10.1093/infdis/149.3.363. [DOI] [PubMed] [Google Scholar]
- Steplewski Z., Spira G., Blaszczyk M., Lubeck M. D., Radbruch A., Illges H., Herlyn D., Rajewsky K., Scharff M. Isolation and characterization of anti-monosialoganglioside monoclonal antibody 19-9 class-switch variants. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8653–8657. doi: 10.1073/pnas.82.24.8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira P., Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988 Feb;18(2):313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- Waldor M. K., Mitchell D., Kipps T. J., Herzenberg L. A., Steinman L. Importance of immunoglobulin isotype in therapy of experimental autoimmune encephalomyelitis with monoclonal anti-CD4 antibody. J Immunol. 1987 Dec 1;139(11):3660–3664. [PubMed] [Google Scholar]


