Abstract
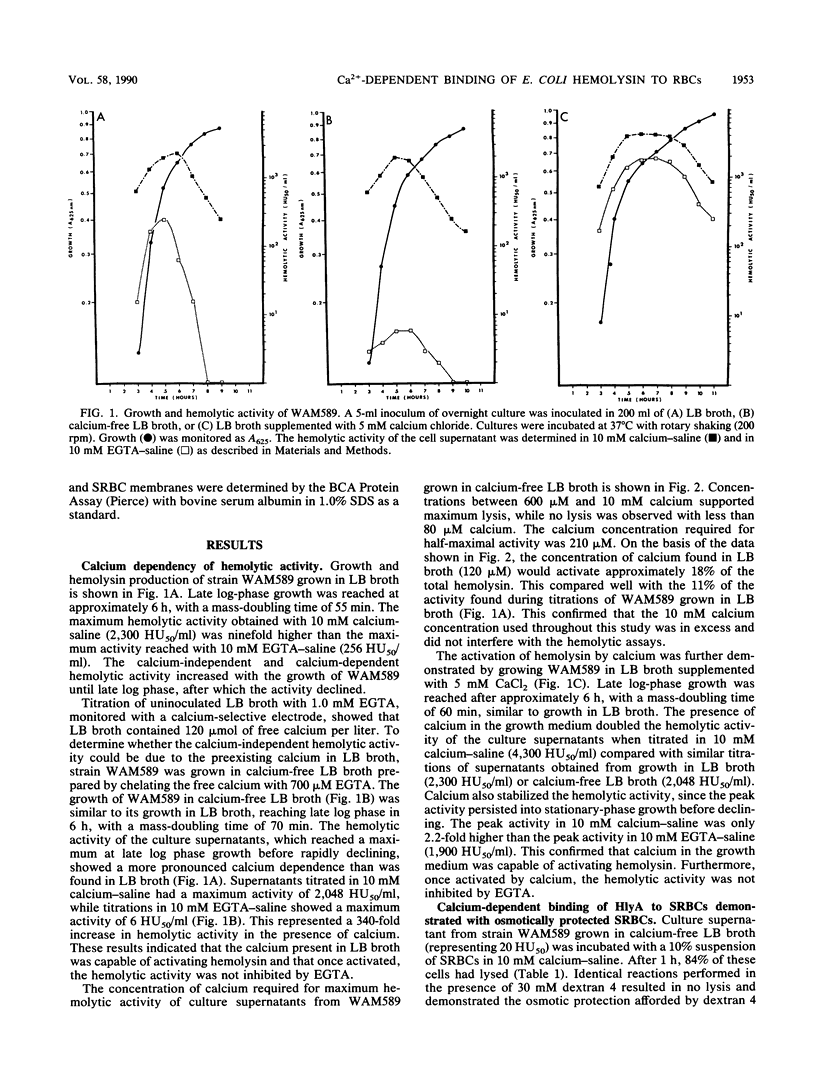
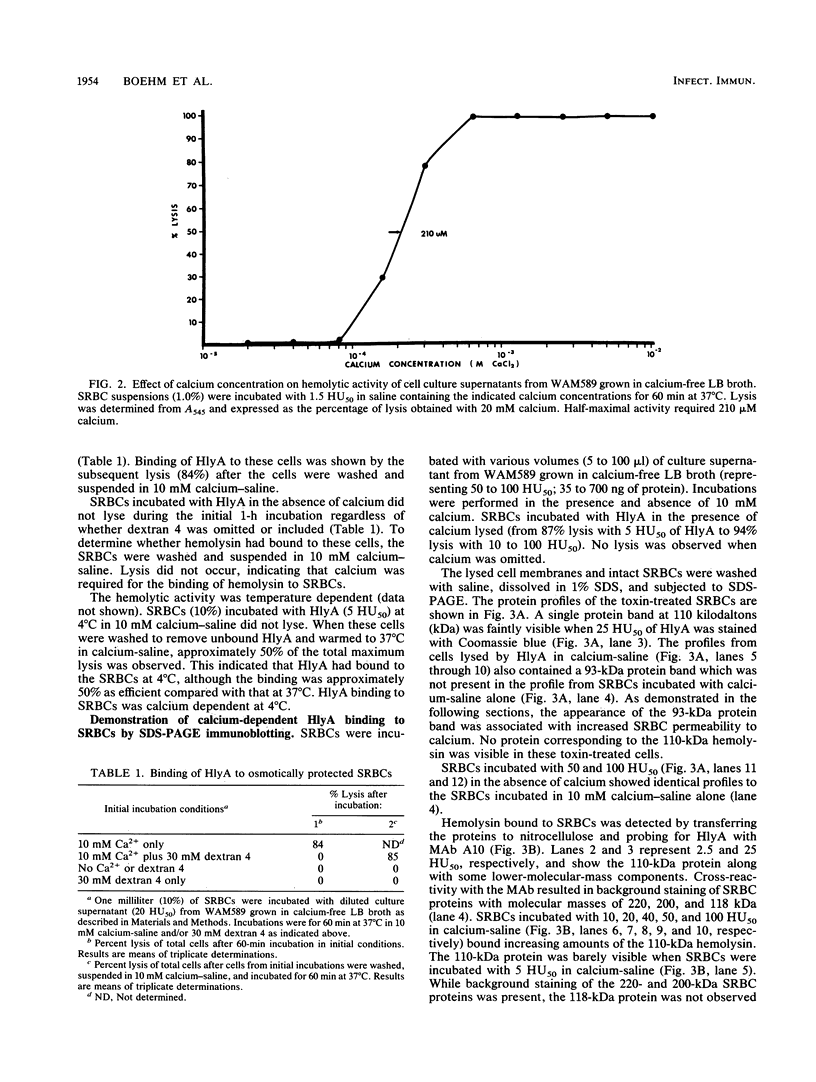
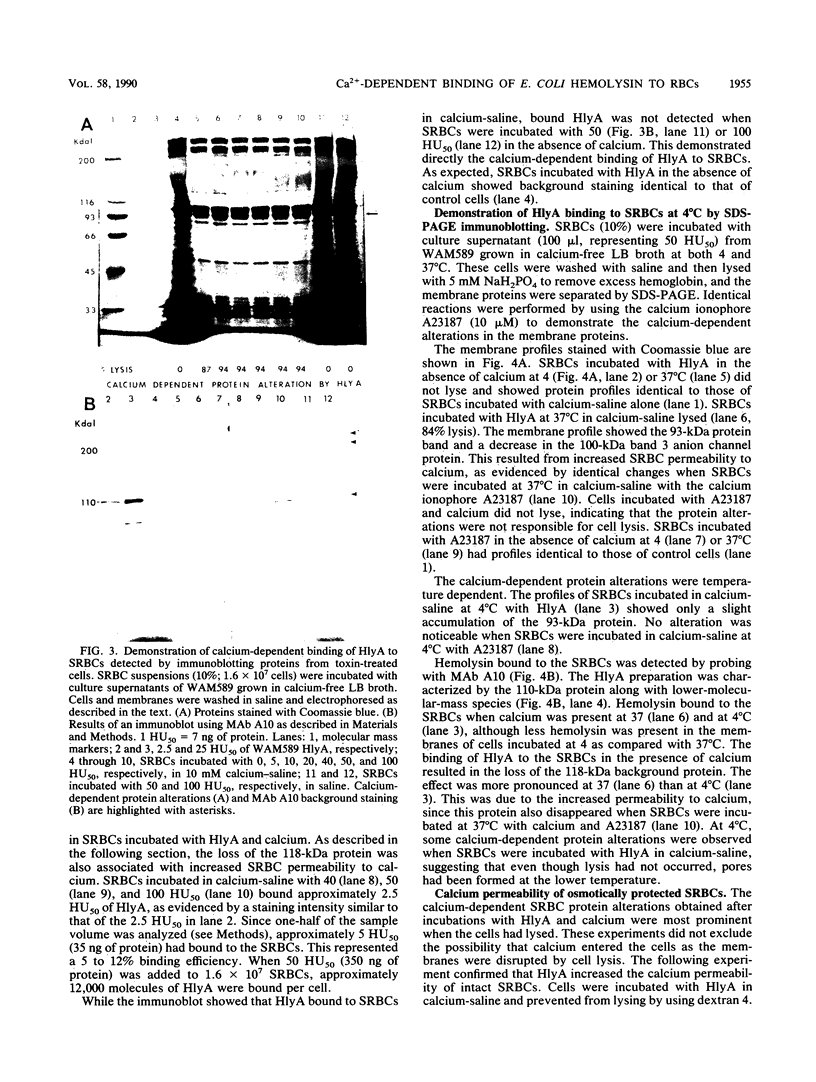
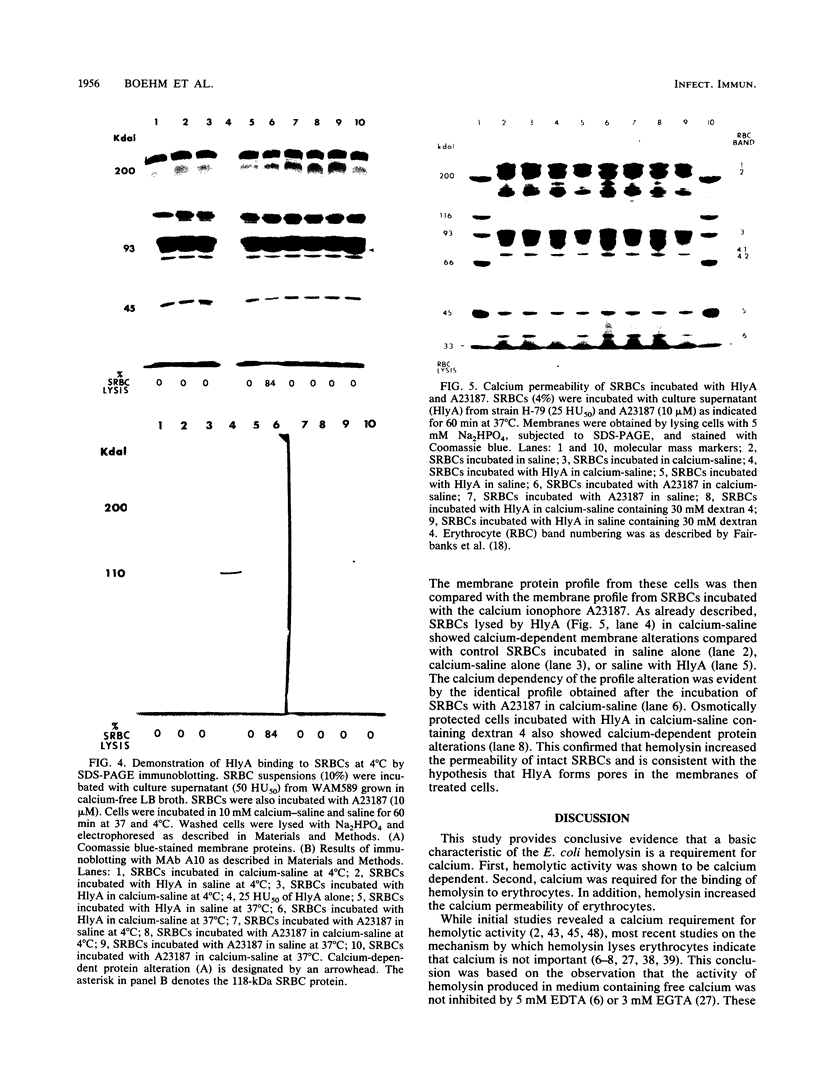
The calcium requirement for hemolytic activity of Escherichia coli hemolysin was investigated by using hemolytic assays and immunoblotting of toxin-treated erythrocytes. The hemolytic activity of cell culture supernatants obtained during growth of E. coli in Luria-Bertani (LB) broth or calcium-free LB broth was calcium dependent. The hemolytic activity of culture supernatants obtained during growth in LB broth supplemented with calcium was calcium independent. Osmotic protection experiments using Dextran 4 to prevent cell lysis indicated that calcium was required for the binding of hemolysin to erythrocytes at both 4 and 37 degrees C. The binding efficiency at 4 degrees C was 50% of that occurring at 37 degrees C. The calcium-dependent binding was confirmed by immunoblotting saline-washed, toxin-treated erythrocytes with a monoclonal antibody after sodium dodecyl sulfate-polyacrylamide gel electrophoresis separation of membrane proteins. Bound hemolysin increased the calcium permeability of the cell membranes as evidenced by calcium-induced membrane protein alterations. The alterations in membrane proteins did not directly cause lysis of the cells. The results were consistent with a mechanism of lysis involving the formation of cation-selective pores in the membranes of target cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W., Cadman S. Calcium-induced erythrocyte membrane changes. The role of adsorption of cytosol proteins and proteases. Biochim Biophys Acta. 1979 Feb 20;551(1):1–9. doi: 10.1016/0005-2736(79)90348-1. [DOI] [PubMed] [Google Scholar]
- BAMFORTH J., DUDGEON J. A. The haemolytic activity of Bact. coli. J Pathol Bacteriol. 1952 Oct;64(4):751–761. doi: 10.1002/path.1700640409. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Benz R., Schmid A., Wagner W., Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989 Mar;57(3):887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Greulich S., Muhly M., Eberspächer B., Becker H., Thiele A., Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989 Mar 1;169(3):737–754. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by pore-forming bacterial cytolysins. Microb Pathog. 1986 Feb;1(1):5–14. doi: 10.1016/0882-4010(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Bohach G. A., Snyder I. S. Chemical and immunological analysis of the complex structure of Escherichia coli alpha-hemolysin. J Bacteriol. 1985 Dec;164(3):1071–1080. doi: 10.1128/jb.164.3.1071-1080.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Cytotoxic activity of partially purified Escherichia coli alpha haemolysin. J Med Microbiol. 1982 Feb;15(1):11–21. doi: 10.1099/00222615-15-1-11. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte function in vitro. Infect Immun. 1982 Sep;37(3):966–974. doi: 10.1128/iai.37.3.966-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982 May;36(2):455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M., Ewins S. P. Properties of strains of Escherichia coli isolated from a variety of sources. J Med Microbiol. 1975 Feb;8(1):107–111. doi: 10.1099/00222615-8-1-107. [DOI] [PubMed] [Google Scholar]
- Croall D. E., Morrow J. S., DeMartino G. N. Limited proteolysis of the erythrocyte membrane skeleton by calcium-dependent proteinases. Biochim Biophys Acta. 1986 Jul 16;882(3):287–296. doi: 10.1016/0304-4165(86)90250-3. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Eberspächer B., Hugo F., Bhakdi S. Quantitative study of the binding and hemolytic efficiency of Escherichia coli hemolysin. Infect Immun. 1989 Mar;57(3):983–988. doi: 10.1128/iai.57.3.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Welch R. A. Alterations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5269–5273. doi: 10.1073/pnas.85.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeberg O. V. In vitro cytotoxic effect of alpha-hemolytic and nonhemolytic Escherichia coli on human blood granulocytes and monocytes. Acta Pathol Microbiol Immunol Scand B. 1987 Aug;95(4):219–225. doi: 10.1111/j.1699-0463.1987.tb03116.x. [DOI] [PubMed] [Google Scholar]
- Gray L., Mackman N., Nicaud J. M., Holland I. B. The carboxy-terminal region of haemolysin 2001 is required for secretion of the toxin from Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):127–133. doi: 10.1007/BF02428042. [DOI] [PubMed] [Google Scholar]
- Gross-Weege W., König W., Scheffer J., Nimmich W. Induction of histamine release from rat mast cells and human basophilic granulocytes by clinical Escherichia coli isolates and relation to hemolysin production and adhesin expression. J Clin Microbiol. 1988 Sep;26(9):1831–1837. doi: 10.1128/jcm.26.9.1831-1837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S. E., Mulcahy P. F., Louis C. F. Effect of Escherichia coli hemolysin on permeability of erythrocyte membranes to calcium. Toxicon. 1986;24(6):559–566. doi: 10.1016/0041-0101(86)90176-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen S. E., Mulcahy P. F., Wu G. K., Louis C. F. Calcium accumulation in human and sheep erythrocytes that is induced by Escherichia coli hemolysin. Toxicon. 1983;21(5):717–727. doi: 10.1016/0041-0101(83)90277-5. [DOI] [PubMed] [Google Scholar]
- Keane W. F., Welch R., Gekker G., Peterson P. K. Mechanism of Escherichia coli alpha-hemolysin-induced injury to isolated renal tubular cells. Am J Pathol. 1987 Feb;126(2):350–357. [PMC free article] [PubMed] [Google Scholar]
- King L. E., Jr, Morrison M. Calcium effects on human erythrocyte membrane proteins. Biochim Biophys Acta. 1977 Nov 15;471(1):162–168. doi: 10.1016/0005-2736(77)90404-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linggood M. A., Ingram P. L. The role of alpha haemolysin in the virulence of Escherichia coli for mice. J Med Microbiol. 1982 Feb;15(1):23–30. doi: 10.1099/00222615-15-1-23. [DOI] [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Lowe-Krentz L. Formation of gamma-glutamyl-epsilon-lysine bridges between membrane proteins by a Ca2+-regulated enzyme in intact erythrocytes. J Supramol Struct. 1978;9(3):427–440. doi: 10.1002/jss.400090313. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Vogel M., Goebel W. Mutations affecting activity and transport of haemolysin in Escherichia coli. Mol Gen Genet. 1987 Feb;206(2):238–245. doi: 10.1007/BF00333579. [DOI] [PubMed] [Google Scholar]
- Mackman N., Baker K., Gray L., Haigh R., Nicaud J. M., Holland I. B. Release of a chimeric protein into the medium from Escherichia coli using the C-terminal secretion signal of haemolysin. EMBO J. 1987 Sep;6(9):2835–2841. doi: 10.1002/j.1460-2075.1987.tb02580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Secretion of haemolysin by Escherichia coli. Curr Top Microbiol Immunol. 1986;125:159–181. doi: 10.1007/978-3-642-71251-7_10. [DOI] [PubMed] [Google Scholar]
- Menestrina G. Escherichia coli hemolysin permeabilizes small unilamellar vesicles loaded with calcein by a single-hit mechanism. FEBS Lett. 1988 May 9;232(1):217–220. doi: 10.1016/0014-5793(88)80420-4. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Mackman N., Holland I. B., Bhakdi S. Escherichia coli haemolysin forms voltage-dependent ion channels in lipid membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):109–117. doi: 10.1016/0005-2736(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Regulation of haemolysin synthesis in E. coli determined by HLY genes of human origin. Mol Gen Genet. 1985;199(1):111–116. doi: 10.1007/BF00327519. [DOI] [PubMed] [Google Scholar]
- Rennie R. P., Freer J. H., Arbuthnott J. P. The kinetics of erythrocyte lysis by Escherichia coli haemolysin. J Med Microbiol. 1974 May;7(2):189–195. doi: 10.1099/00222615-7-2-189. [DOI] [PubMed] [Google Scholar]
- SMITH H. W. The haemolysins of Escherichia coli. J Pathol Bacteriol. 1963 Jan;85:197–211. doi: 10.1002/path.1700850119. [DOI] [PubMed] [Google Scholar]
- Shields M., La Celle P., Waugh R. E., Scholz M., Peters R., Passow H. Effects of intracellular Ca2+ and proteolytic digestion of the membrane skeleton on the mechanical properties of the red blood cell membrane. Biochim Biophys Acta. 1987 Nov 27;905(1):181–194. doi: 10.1016/0005-2736(87)90022-8. [DOI] [PubMed] [Google Scholar]
- Short E. C., Kurtz H. J. Properties of the Hemolytic Activities of Escherichia coli. Infect Immun. 1971 May;3(5):678–687. doi: 10.1128/iai.3.5.678-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The toxic role of alpha-haemolysin in the pathogenesis of experimental Escherichia coli infection in mice. J Gen Microbiol. 1985 Feb;131(2):395–403. doi: 10.1099/00221287-131-2-395. [DOI] [PubMed] [Google Scholar]
- Snyder I. S., Zwadyk P. Some factors affecting production and assay of Escherichia coli haemolysins. J Gen Microbiol. 1969 Jan;55(1):139–143. doi: 10.1099/00221287-55-1-139. [DOI] [PubMed] [Google Scholar]
- Springer W., Goebel W. Synthesis and secretion of hemolysin by Escherichia coli. J Bacteriol. 1980 Oct;144(1):53–59. doi: 10.1128/jb.144.1.53-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Falkow S. Characterization of Escherichia coli hemolysins conferring quantitative differences in virulence. Infect Immun. 1984 Jan;43(1):156–160. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]