Abstract
Receptor desensitization involving receptor phosphorylation and subsequent βArrestin (βArr) recruitment has been implicated in the tolerance development mediated by μ-opioid receptor (OPRM1). However, the roles of receptor phosphorylation and βArr on morphine-induced OPRM1 desensitization remain to be demonstrated. Using OPRM1-induced intracellular Ca2+ ([Ca2+]i )release to monitor receptor activation, as predicted, [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO), induced OPRM1 desensitization in a receptor phosphorylation- and βArr-dependent manner. The DAMGO-induced OPRM1 desensitization was attenuated significantly when phosphorylation deficient OPRM1 mutants or Mouse Embryonic Fibroblast (MEF) cells from βArr1 and 2 knockout mice were used in the studies. Specifically, DAMGO-induced desensitization was blunted in HEK293 cells expressing the OPRM1S375A mutant and was eliminated in MEF cells isolated from βArr2 knockout mice expressing the wild type OPRM1. However, although morphine also could induce a rapid desensitization on [Ca2+]i release to a greater extent than that of DAMGO and could induce the phosphorylation of Ser375 residue, morphine-induced desensitization was not influenced by mutating the phosphorylation sites or in MEF cells lacking βArr1 and 2. Hence, morphine could induce OPRM1 desensitization via pathway independent of βArr, thus suggesting the in vivo tolerance development to morphine can occur in the absence of βArr.
Keywords: μ-opioid receptor (OPRM1); phosphorylation; βArrestin(βArr); desensitization; morphine; [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin(DAMGO)
Introduction
Chronic or repetitive usage of opioid analgesic such as morphine results in the development of tolerance, which largely prevents the clinical usage of morphine. However, the exact molecular mechanism of morphine tolerance development has not been demonstrated unequivocally. Opioid receptor desensitization has been suggested to be closely related to the in vivo tolerance development [1–3] . Since μ-opioid receptor (OPRM1) has been demonstrated to be the major opioid receptor to mediate the analgesia and tolerance effects of morphine by using receptor null mice [4, 5], studies on cellular mechanism of opioid tolerance have focused mostly on OPRM1 desensitization.
Currently, the mechanism of GPCR desensitization is based mainly on the involvement of GRK and βArrs. After agonist binds to the receptor, GRK will mediate receptor phosphorylation and subsequently increase the affinity of agonist-receptor complex for the cytosolic protein βArr. Translocation of βArr to receptor disrupts receptor-G protein coupling and dampens the receptor signal transduction processes [6]. This GPCR regulatory mechanism has been demonstrated with various receptors such as β2-adrenergic receptor, m2-muscarinic receptors, dopamine D1A receptor [7–10] among other GPCRs within the rhodopsin subfamily of GPCR. Likewise, agonist-mediated phosphorylation of δ-opioid receptor (OPRD1) has been shown to lead to βArrs recruitment and eventual OPRD1 desensitization [11–13]. However, the βArr involvement in morphine-induced OPRM1 desensitization is equivocal. On one hand, the involvement of βArr in morphine-induced in vivo tolerance development was demonstrated with the βArr2 null mice studies with the hot plate but not with the tail-flick antinociceptive assays [1]. Also, the ability of morphine to induce in vitro tolerance development was impeded in the GRK3 knockout mice [14]. These studies supported a role of βArr in morphine-induced tolerance. On the other hand, morphine was unable to induce OPRM1 phosphorylation and βArrs recruitment [3, 15, 16]. In addition, when Gαi-mediated inhibition of adenylyl cyclase activity was monitored, OPRM1 desensitization was observed only after several hours of DAMGO pretreatment while receptor phosphorylation and βArr recruitment occurred within minutes [8, 17]. Moreover, the inability of GRKs and βArr2 overexpression to affect OPRM1 desensitization in various cell types further suggested a lack of correlation between OPRM1 desensitization and GRK-mediated receptor phosphorylation and βArrs recruitment [16, 18, 19]. These divergent observations of OPRM1 desensitization might be due to the relatively weak interaction between OPRM1, GRKs and βArr when compared to DOR [20].
In order to address the mechanism of morphine-induced receptor desensitizaiton, it is critical to have a sensitive experimental measurement to indicate changes in receptor activity within minutes. Previous studies indicated that Gαi is more efficient in transducing opioid receptor signals than Gβγ-subunits [21]. This is probably the reason why opioid receptor desensitized in hours when Gαi-mediated inhibition of adenylyl cyclase activity was measured, while desensitized in minutes when Gβγ-mediated activation of potassium channel was monitored [22]. Although the [Ca2+]i release has been shown to be mediated either by the direct activation of phospholipase Cβ [23, 24], or by the co-activation of Gq-coupled receptors [25, 26], it is unequivocal that opioid receptor mediated this response via the Gβγ subunits [23, 24]. Hence, in current study, the role of receptor phosphorylation in morphine- and DAMGO-induced OPRM1 desensitization was examined by monitoring opioid receptor-mediated [Ca2+]i release in HEK293 cells expressing wild type or phosphorylation deficient OPRM1 mutants. The role of βArr in morphine- and DAMGO-induced receptor desensitization was determined with mouse embryonic fibroblast (MEF) cells isolated from wild type and βArr deficient mice. It can be shown that in contrast to DAMGO, morphine could induce OPRM1 desensitization without receptor phosphorylation and the involvement of βArr.
Materials and Methods
Cell Culture and Chemicals
Hemagglutinin (HA) tagged μ-opioid receptor (HA-OPRM1), HA-OPRM1 in which Ser363, Thr370 or Ser375 residues was individually mutated to Ala (HA- OPRM1S363A, HA- OPRM1T370A, HA- OPRM1S375A) and HA-OPRM1 with Ser363, Thr370 and Ser375 residues mutated to Ala (HA-OPRM1363/370/375) were stably expressed in HEK293 cells as described before [27]. Cells were grown in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) with Earle’s salt supplemented with 10% fetal bovine serum and 200 ng/ml G418 sulfate. Receptor expression level were determined by binding assays. Wild type, β-arrestin2 null (βArr2−/−) and β-arrestin1 and 2 null (βArr1/2−/−) Mouse Embryonic Fibroblasts (MEF) cells (generous gifts from Dr. R. Lefkowitz, Duke University, NC) were cultured in DMEM supplemented with 10% fetal bovine serum. Normally, cells were cultured for 24 hr before agonist treatment. Effectene (QIAgen, Santa Clanta, CA) was used to transfect βArr2 to HEK293 cells and Adenovirus was used to express OPRM1 in MEF cells after 24 hr of culturing. The cells were cultured for additional 48 hr after the transfection or virus infection. Then agonists or inhibitors were added for desired concentration and time as described in Figure legend.
Adenoviral infection of MEF cells
The titer of the adenovirus containing OPRM1 (Ad-OPRM1) was determined to be ~2.5×109 infectious units (IU)/ml. MEF cells were grown in DMEM with 10% FBS at 6-well-plate to be about 50% confluent. Then the media was removed. Adenovirus containing OPRM1 (Ad-OPRM1) virus was diluted in DMEM with 2%FBS and added to the wells. Multiplicity of infection (MOI) was determined by making virus dose and receptor expressing level curve. Desired MOI was used to reach the approximated same receptor expression level. After 1and half hour, DMEM with 10%FBS was added back to the wells. Cells were incubated at 37°C for 48hours before the assays were carried out.
Intracellular Ca2+ measurement
One day before the assay, CORNING® black with clear flat bottom 96-well-assay plate was coated with poly-L-Lysine. HEK293 cells or MEF cells, which were grown in MEM or DMEM as described previously, were suspended in the same medium and plated at a density of ~3×104 cells/well in 150µl medium. HEK293 and MEF cells were incubated in a humidified atmosphere of 5% or 10% CO2 respectively at 37°C overnight so as to reach an 80~90% confluent cell monolayer before assay. At the day of assay, 100µl medium/well was removed from plate. To each well, 50µl FLIPR® calcium assay reagent (Molecular Devices Corp) dissolved in 1x reagent buffer (1×HANKs buffer with 20mM HEPES), pH 7.4, with 5mM probenecid was added and the plate was incubated at 37°C for 1 hour. Agonists, inhibitors and other reagents were dissolved in the assay buffer (HBSS: KCl 5mM, KH2PO4 0.3mM, NaCl 138mM, NaHCO3 4mM, Na2HPO4 0.3mM, D-glucose 5.6mM, with additional 20mM HEPES, 2.5mM probenecid and 13mM CaCl2). Using a FLEXstation (Molecular Devices Corp.), the [Ca2+]i fluorescence increases after robotic injections of agonists, inhibitors or other reagents were monitored every 1.52 sec intervals with excitation wavelength at 485 nm and with emission wavelength at 525nm. The [Ca2+]i release normally reached its maximum 15 sec after agonist injection and returned to baseline within 30 sec after injection. The [Ca2+]i fluorescence was measured up to 90 sec after agonist injection. The fluorescence intensity from 3 to 4 wells of cells were averaged and the relative amount of [Ca2+]i release was determined by integrating the area under the peak of the [Ca2+]i fluorescence averages.
Immunoprecipitation
Cells were extracted with cell lysis buffer (1% Brij-98, 50 mM Tris-HCl pH 7.5, 150mM NaCl, 0.25% sodium deoxycholate, 0.1%, 50mM NaF, 1mM dithiothreitol, 0.5mM phenylmethylsulfonyl fluoride, 50mM sodium pyrophosphate, 10mM sodium vanadate and 1×protease inhibitor cocktail from Roche, Indianapolis, IN). After centrifugation at 12,000xg for 5min, the supernatant was immunoprecipitated with rabbit anti HA (Covance, NJ) and rProtein G agarose beads (Invitrogen, CA) at 4°C overnight. Then the beads were washed six times with cell lysis buffer and were extracted with SDS-PAGE sample buffer. Approximated equal amount of protein was resolved by SDS-PAGE and was analyzed as described above. For the measurement of OPRM1 phosphorylation, the anti-phospho-Ser375-OPRM1 antibody (P375OPRM1) (Cell Signaling, MA) or the total Phospho-Ser/Thr antibody (Pan) (Zymed laboratories, CA) was used and was normalized to the protein concentration of the samples.
Opioid receptor binding
48 hr after the transfection or virus infection, cells were harvested and resuspended in 25mM HEPES buffer, PH 7.6, containing 5mM MgCl2 at 24°C for 90 min. After the protein concentrations of the pellets were determined by the Lowry method, [3H]diprenorphine (2 nM) binding in the presence or absence of 10 µM naloxone were added together so as to determine the specific binding.
Results
OPRM1-mediated intracellular Ca2+ release in HEK293 cells required P2Y receptor co-activation
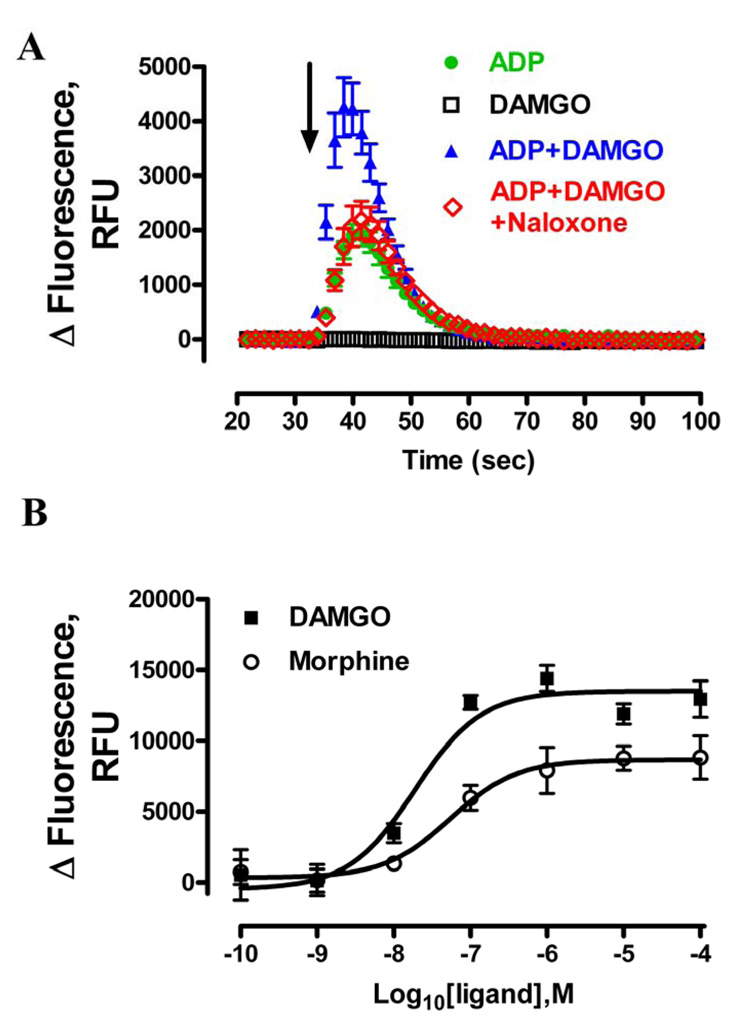
In current study, a FLIPR® calcium assay kit was used to detect [Ca2+]i release. Unlike the Fluo-3 and Fluo-4, the FLIPR® calcium assay reagent is a no-wash fluorescent calcium indicator with minimal background fluorescence signal. Thus this assays limited perturbation and stress on cells. After loading the dye for 1hr, the baseline was determined by recording the Ca2+ fluorescence for 30 sec at 525nm before drug injection. Consistent with previous report [28, 29], challenging HEK293-OPRM1 cells with DAMGO or morphine alone did not induce Ca2+ release (Fig. 1A and supplementary Fig. 1). In contrast, ADP, a Gq-coupled P2Y receptor agonist, evoked a robust Ca2+ release (Figure 1A) in a concentration-dependent manner (EC50=9.4 ± 4.0 µM) (Fig. 3A). This ADP-induced Ca2+ release was blocked by the P2Y general antagonist suramin and as expected, it was not affected by the removal of the extracellular Ca2+, which indicated the Ca2+ was mobilized from [Ca2+]i pool (data not shown). When DAMGO or morphine was used together with ADP, ADP-induced [Ca2+]i release was greatly enhanced (Fig. 1A and SD Fig. 1). Moreover, this potentiation was also observed in the absence of extracellular Ca2+ pool (data not shown) and was due to the activation of OPRM1. OPRM1 antagonist naloxone attenuated 1µM DAMGO-potentiated [Ca2+]i release with EC50 at 710 ± 140nM (data not shown).
Fig. 1. Activation of OPRM1 induced [Ca2+]i release in HEK293 cells.
A, OPRM1-mediated [Ca2+]i release required P2Y receptor co-activation. Data shows the real time intracellular fluorescence change in raw fluorescence unit (RFU). HEK293 cells were cultured as described in materials and methods. Fluorescence dye to detect free [Ca2+]i was added 1 hour before compound injection. After 30 second baseline reading, as indicated by the arrow, HEK293 cells expressing OPRM1 were injected with 200nM ADP ( ), 1µM DAMGO (□), 200nM ADP with 1µM DAMGO (
), 1µM DAMGO (□), 200nM ADP with 1µM DAMGO ( ), 200nM ADP, 1µM DAMGO and 30µM Naloxone (
), 200nM ADP, 1µM DAMGO and 30µM Naloxone ( ) respectively. B, The concentration-response curves of DAMGO (■) and morphine (○) were determined in the presence of 200nM ADP. Total fluorescence response change induced by ADP or by ADP with OPRM1 ligands was quantified by analyzing the areas under the curves with Prism program. ADP response was then subtracted from the response in the presence of ADP and OPRM1 ligands to obtain the DAMGO and morphine responses.
) respectively. B, The concentration-response curves of DAMGO (■) and morphine (○) were determined in the presence of 200nM ADP. Total fluorescence response change induced by ADP or by ADP with OPRM1 ligands was quantified by analyzing the areas under the curves with Prism program. ADP response was then subtracted from the response in the presence of ADP and OPRM1 ligands to obtain the DAMGO and morphine responses.
Fig. 3. Morphine-induced OPRM1 desensitization was resulted from loss of OPRM1 activity.
A, Morphine or DAMGO pretreatment did not affect ADP-mediated response. HEK293 cells expressing wild type OPRM1 were pretreated with 1µM morphine( ), DAMGO(
), DAMGO( ) or HBSS(▲)respectively. In second injection, various concentration of ADP together with 30µM naloxone was injected. Then the concentration-response curves of ADP were determined as described in materials and methods. B, Morphine or DAMGO pretreatment did not alter [Ca2+]i store availability. HEK293 cells expressing wild type OPRM1 were cultured and seeded as described in materials and methods. After 1 hr incubation of fluorescence dye, cells were washed with Ca2+ free HBSS buffer. Then cells were incubated in Ca2+ free HBSS buffer with 1mM EGTA, and were treated with agonists. After cells were pretreated with 1µM morphine, DAMGO, 10µM ADP or HBSS respectively, 1µM thapsigargin was added. Thapsigargin-induced total fluorescence change in second injection was calculated as described in materials and methods; the data were expressed as the raw fluorescence units in bar graph. Student t-test was used to compare the data in treated groups and control group. ** denotes p< 0.01.
) or HBSS(▲)respectively. In second injection, various concentration of ADP together with 30µM naloxone was injected. Then the concentration-response curves of ADP were determined as described in materials and methods. B, Morphine or DAMGO pretreatment did not alter [Ca2+]i store availability. HEK293 cells expressing wild type OPRM1 were cultured and seeded as described in materials and methods. After 1 hr incubation of fluorescence dye, cells were washed with Ca2+ free HBSS buffer. Then cells were incubated in Ca2+ free HBSS buffer with 1mM EGTA, and were treated with agonists. After cells were pretreated with 1µM morphine, DAMGO, 10µM ADP or HBSS respectively, 1µM thapsigargin was added. Thapsigargin-induced total fluorescence change in second injection was calculated as described in materials and methods; the data were expressed as the raw fluorescence units in bar graph. Student t-test was used to compare the data in treated groups and control group. ** denotes p< 0.01.
The level of the OPRM1-mediated potentiation was related to the initial ADP response. There was no significant potentiation of [Ca2+]i release when ADP maximally induced the [Ca2+]i store to release, suggesting OPRM1 and ADP were inducing the release of Ca2+ from the same intracellular Ca2+ pools. On the other hand, OPRM1 did not potentiate the [Ca2+]i release when ADP did not evoke measurable [Ca2+]i to release. Thus, in order to optimize the opioid agonist-mediated [Ca2+]i release, 0.2 µM ADP was used to initiate [Ca2+]i release. The potentiation of ADP response induced either by DAMGO or by morphine was ligand concentration-dependent (Fig. 1B). The EC50 value of DAMGO to potentiate the 0.2 µM ADP response was 19 ± 6.9 nM, which was significantly different from that of the morphine, 54 ± 9.8 nM (p<0.01, n=4). Furthermore, maximal potentation induced by morphine was only 64 ± 14% of that induced by DAMGO (Fig. 1B), suggesting that morphine is a partial agonist in invoking [Ca2+]i to release.
μ-opioid receptor can be rapidly desensitized by morphine and DAMGO
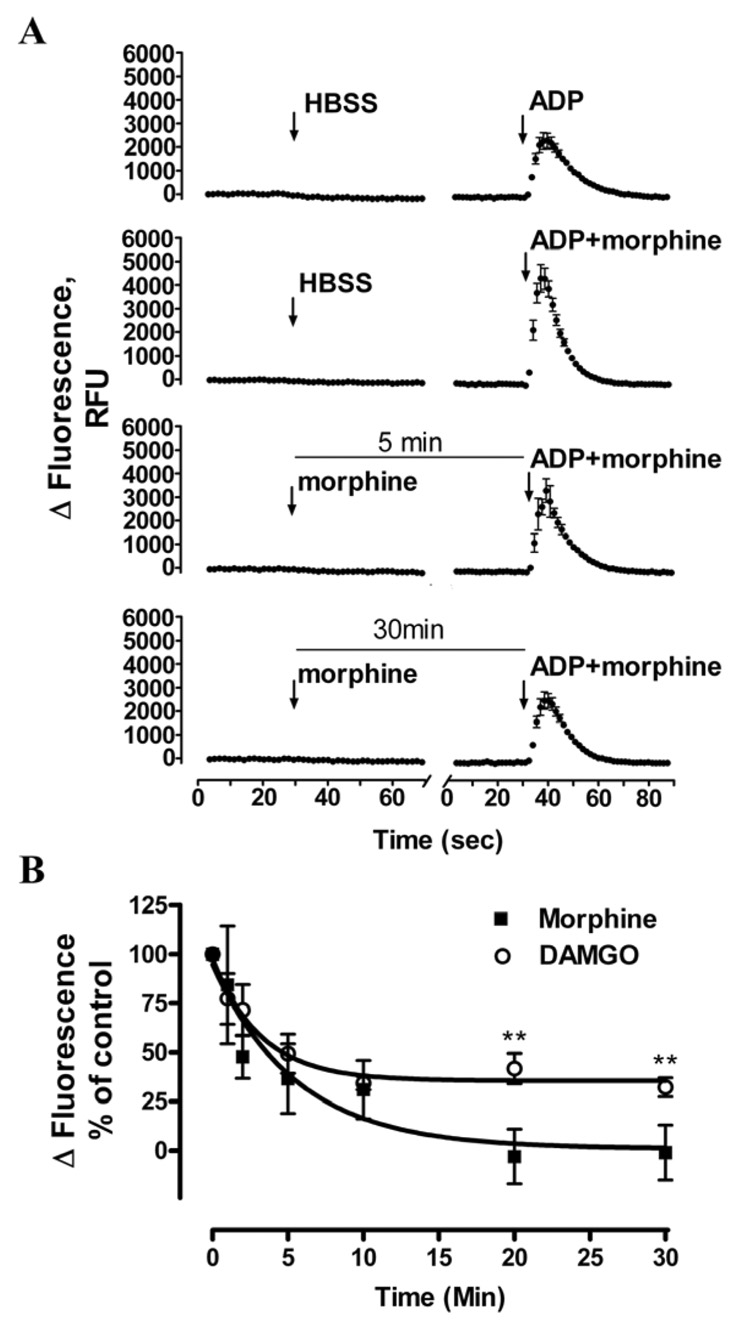
Whether pretreatment of DAMGO or morphine induced OPRM1 rapid desensitization was examined. In the control group, cells were pretreated with buffer, followed by the addition of OPRM1 agonist together with ADP. Subsequent potentiation of ADP-induced [Ca2+]i release by OPRM1 agonist was measured. In the desensitization group, HEK293-OPRM1 cells were pretreated with OPRM1 agonist, followed by the addition of ADP and OPRM1 agonists together in order to achieve the same final concentration of ADP and OPRM1 agonist as in the control groups (Fig. 2A). As shown in Fig. 2A, the OPRM1-mediated potentiation of ADP-induced [Ca2+]i release was significantly decrease in a time-dependent manner by morphine treatment. In contrast to other reports which suggested that morphine was a poor desensitization inducer [30], morphine pretreatment induced rapid OPRM1 desensitization in the [Ca2+]i release. After pretreating the HEK293 cells with 100nM morphine for 5min, the morphine-mediated potentiation of ADP-induced [Ca2+]i release decreased to 37±15% of control. Extending the morphine pretreatment time to 30min eliminated the potentiation of [Ca2+]i release (Fig. 2B). DAMGO-induced OPRM1 desensitization was also determined to be rapid. Although the t1/2 of 100nM DAMGO- and morphine-induced OPRM1 desensitization was 2.2 ± 0.6 min and 3.8±1.1 min respectively, 100 nM morphine-induced maximum desensitization level was 0.8 ± 12% of control whereas the 100 nM DAMGO-induced maximal desensitization level was 35 ± 9% of control (n = 4; p < 0.01). In addition, when HEK293 cells were pretreated with 1 µM morphine, which was an equivalent to 100nM DAMGO at their EC90 concentration to induced intracellular calcium release, the t1/2 of morphine-induced OPRM1 desensitization was 0.8 ± 0.4 min, which was significantly faster than that of the DAMGO (n = 4; p < 0.01) (data not shown).
Fig. 2. OPRM1 agonists induced rapid OPRM1 desensitization in HEK293 cells.
A, 100nM morphine-pretreatment rapidly reduced future OPRM1 activation. In the first injection, 100nM morphine in HBSS buffer was added into pretreated groups while HBSS was added into control groups. In the second injection, 900nM morphine and 200nM ADP were added into pretreated groups, while 1µM morphine and 200nM ADP were added into control groups; thus the same final concentration of morphine and ADP in both control and pretreated groups was achieved. Then the OPRM1-mediated potentiation of ADP-induced [Ca2+]i release was measured. B, Morphine and DAMGO induced OPRM1 rapid desensitization. The ability of 100nM DAMGO(○) and morphine(■) to induced desensitization of wild type OPRM1 in HEK293 cells was examined as described in Fig. 2A. Total [Ca2+]i response of the second injection was quantitatively analyzed as described in Methods and legend of Figure 1. The total response in control groups of 200nM ADP and 1µM DAMGO-induced in second injection was referred as 100%; and data were expressed as the percentage of the response in the pretreated group as compare to the control group. ** denotes p< 0.01.
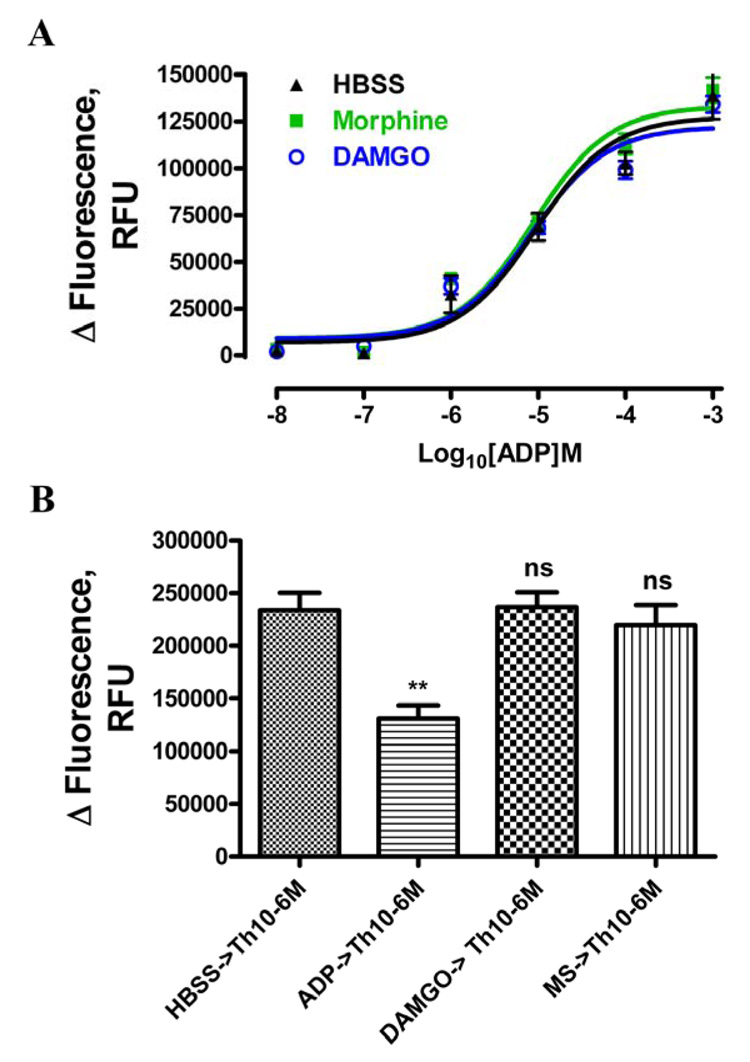
Such reduction in the [Ca2+]i release could be due to the attenuation of ADP activation of the P2Y receptor. In order to exclude this possibility, ADP-concentration-dependent [Ca2+]i release were determined after OPRM1 agonists pretreatment. 30µM naloxone was added together with ADP to block further OPRM1 activation. Pretreatment of HEK293 cells with 1µM morphine for 5min did not alter the ADP response (EC50 = 9.4 ± 4.0µM or 8.9 ± 2.7µM without or with morphine pretreatment respectively, Fig. 3A). Similarly, pretreatment with 1µM DAMGO did not alter the ADP response also (EC50 = 8.5 ± 3.8µM, Fig. 3A). Additionally, the decrease of morphine-induced intracellular release was not due to a reduction in [Ca2+]i store availability. The HEK-OPRM1 cells were washed with Ca2+ free buffer containing 1mM EGTA to block Ca2+ influx. Although 10 µM ADP pretreatment for 90sec significantly decreased the 1 µM thapsigargin-induced [Ca2+]i release (n = 3; p < 0.01) [31], 90sec pretreatments of 10µM morphine or DAMGO did not alter thapsigargin-induced [Ca2+]i release (Fig. 3B). These observations suggested that the decrease in OPRM1-induced [Ca2+]i release after agonist pretreatment was caused by OPRM1 desensitization.
Effect of receptor phosphorylation on OPRM1 desensitization
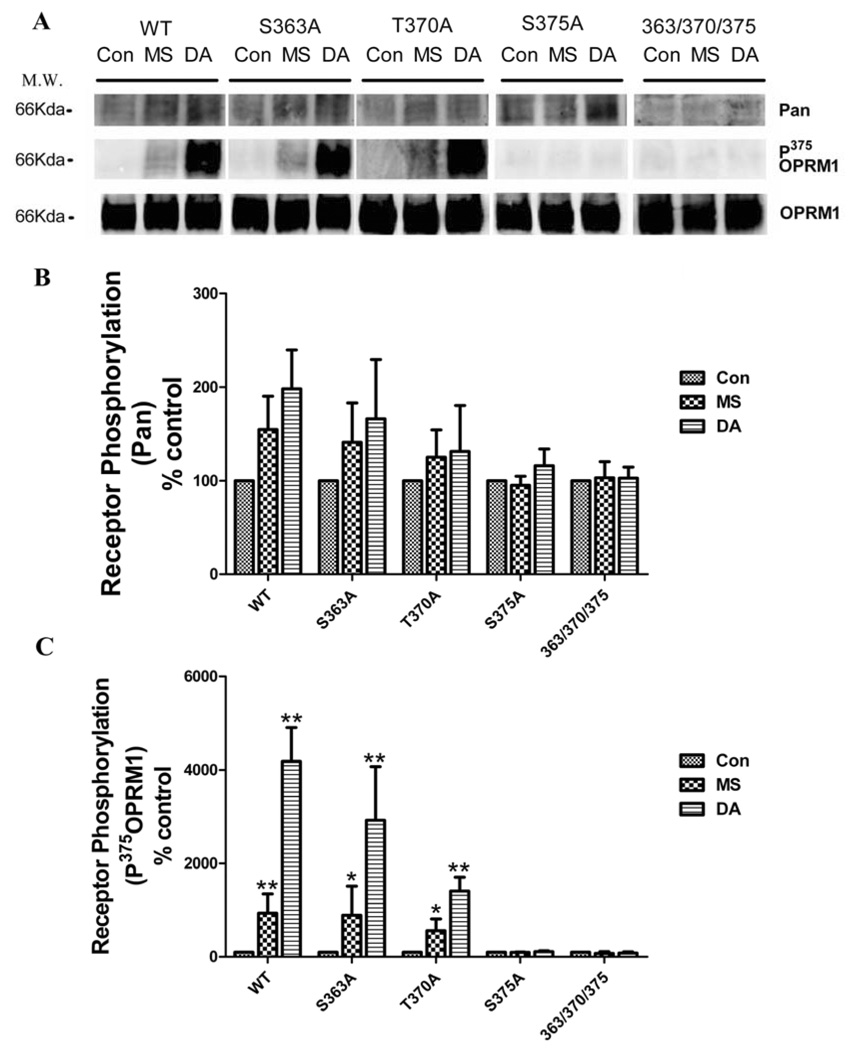
Current hypothesis suggests that homologous desensitization depends on GRK-mediated receptor phosphorylation. There are three serine or threonine residues in carboxyl tail of OPRM1 that have been shown to be phosphorylated. With the exception of Ser363 that was shown to be phosphorylated in the absence of agonist, OPRM1 phosphorylation was agonist-dependent. DAMGO induced phosphorylation on Thr370 and Ser375 was mediated by GRK [35] and affected the rate and extent of DAMGO-induced receptor internalization significantly [27]. In contrast to DAMGO, morphine only induced OPRM1 phosphorylation on Ser375 to a lesser extent [32]. Thus in order to determine further the underlying relationship between OPRM1 desensitization and receptor phosphorylation, OPRM1 with individual Ser363(OPRM1S363A), Thr370(OPRM1T370A) and Ser375(OPRM1S375A) mutations or phosphorylation deficient OPRM1(OPRM1363/370/375) in which all three phosphorylation residues were mutated to Ala were stably expressed in HEK293 cells and used in current studies.
Basal phosphorylation level of wild type OPRM1 was detected in the absence of agonist. After agonist pretreatment for 5min, wild type OPRM1 phosphorylation level was increased 1.5-folds and 2-folds in the presence of 1 µM morphine and DAMGO respectively. Mutating either Thr370 or Ser375 but not Ser363 to Ala attenuated this agonist-induced OPRM1 phosphorylation. Both basal and agonist-induced phosphorylation was completely blocked in phosphorylation deficient OPRM1 in which all three phosphorylation residues (363/370/375) were mutated (Fig 4), consistent with the previous report using metabolic 32P-labeling of the receptor. Moreover, different from DAMGO, the major phosphorylation residues induced by morphine treatment was Ser375, for mutating this single site completely blocked morphine-induced phosphorylation. However, DAMGO still increased receptor phosphorylation in S375A (Fig 4 A, B). In addition, mutating single Thr370 residue attenuated DAMGO-induced OPRM1 phosphorylation without affecting morphine-induced phosphorylation (Fig 4C).
Fig. 4. OPRM1 agonists induced OPRM1 phosphorylation in HEK293 cells.
HEK 293 cells stably expressing HA-tagged mutant or wild type OPRM1 were pretreated with 1µM morphine or 1µM DAMGO for 5 min. Receptors were immunoprecipitated and receptor phosphorylation was quantitatively analyzed as described in materials and methods. Student t-test was used to compare the data in morphine- or DAMGO-pretreated group to control group. * denotes p < 0.05; ** denotes p< 0.01.
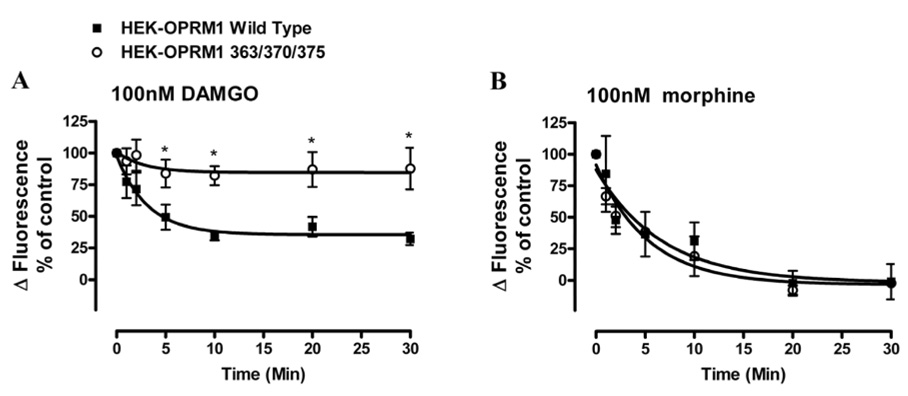
When the agonist-induced receptor desensitization was determined in these OPRM1 mutants, S375A but not S363A or T370A mutation decreased 100nM DAMGO-induced OPRM1 desensitization significantly. In contrast to wild type, S363A or the T370A mutants which reduced the agonist activity by 60–65% after 30 min of DAMGO pretreatment, the DAMGO activity in cells expressing the S375A mutant was reduced by 28% after agonist pretreatment (Table 1). In addition, mutation of the Thr370 and Ser375 or the mutation of all three putative phosphorylation sites resulted in the attenuation of DAMGO-induced desensitization after 5min of 100nM DAMGO pretreatment (p < 0.05, n = 3). Prolonged pretreatment did not further desensitize OPRM1 (363/370/375) activity. After 30min 100nM DAMGO pretreatment, 85 ± 14% of control activity remained (Fig. 5A).
Table 1. Desensitization of wild and mutant types OPRM1 by 100nM DAMGO pretreatment in HEK293 cells.
DAMGO-induced [Ca2+]i release concentration-reponse curves were determined as described in Fig 1B, and EC50 values of DAMGO were calculated by log(agonist)vs. response formula. 100nM DAMGO-induced desensitization in wild type or mutant types OPRM1 was examined and data were analyzed as described in figure legend of Figure 2B. K values of DAMGO-induced OPRM1 desensitization were calculated by one phase exponential decay using the data analysis program GraphPad Prism. Maximum desensitization(des) level data were obtained after 30 min agonist pretreatment. Receptor expression level was determined by radioligand binding experments as described in material and methods. Data were showed as the averages of n≥3 experiments.
| HEK293-OPRM1 | Response | Desensitization | Receptor desensity, (pmol/mg) | |
|---|---|---|---|---|
| EC50,DAMGO (nM) | K, (min−1) | Maximum des level,(%) | ||
| WT | 19 ± 6.9 | 0.31 ± 0.12 | 65 ± 4 | 2.2 ± 0.4 |
| S363A | 60 ± 19 | 0.46 ± 0.32 | 59 ± 5 | 1.8 ± 0.3 |
| T370A | 65 ± 31 | 0.40 ± 0.11 | 60 ± 9 | 1.6 ± 0.3 |
| S375A | 58 ± 53 | 0.37 ± 0.19 | 28 ± 11* | 1.3 ± 0.4 |
| 370/375 | 11 ± 9.1 | 0.41 ± 0.10 | 21 ± 10* | 2.5 ± 0.7 |
| 363/370/375 | 17 ± 3.6 | 0.35 ± 0.18 | 15 ± 14** | 1.7 ± 0.5 |
means ± SEM (n ≥3)
p < 0.05
p < 0.01.
Fig. 5. Effect of receptor phosphorylation on DAMGO- and morphine-induced OPRM1 desensitization in HEK293 cells.
The ability of 100nM DAMGO and morphine to induce desensitization of wild type OPRM1(■) and phosphorylation deficient OPRM1(363/370/375)(○) was examined in HEK293 cells. Total [Ca2+]i response of the second injection was quantitatively analyzed as described in figure legend of Figure 1B. The agonist-induced desensitization rate was calculated as described in the legend of Fig. 2B. Data were showed as the averages of n≥3 experiments in which HEK293 cells pretreated with 100nM DAMGO (panel A) and 100nM morphine (panel B) respectively to induce OPRM1 desensitization. * denotes p < 0.05; and ** denotes p< 0.01.
In contrast to DAMGO, although morphine treatment also induced rapid desensitization, both the rate and extent of OPRM1 desensitization were not affect in the phosphorylation deficient OPRM1 mutants. As shown in Fig. 5B, the ability of 100 nM morphine to induce receptor desensitization as determined by the [Ca2+]i release was identical in HEK293 cells expressing either the wild type or the OPRM1(363/370/375) mutant. Apparently, morphine-induced OPRM1 desensitization did not require the initial phosphorylation of the receptor.
Effect of β-arrestins on morphine- or DAMGO-induced OPRM1 desensitization
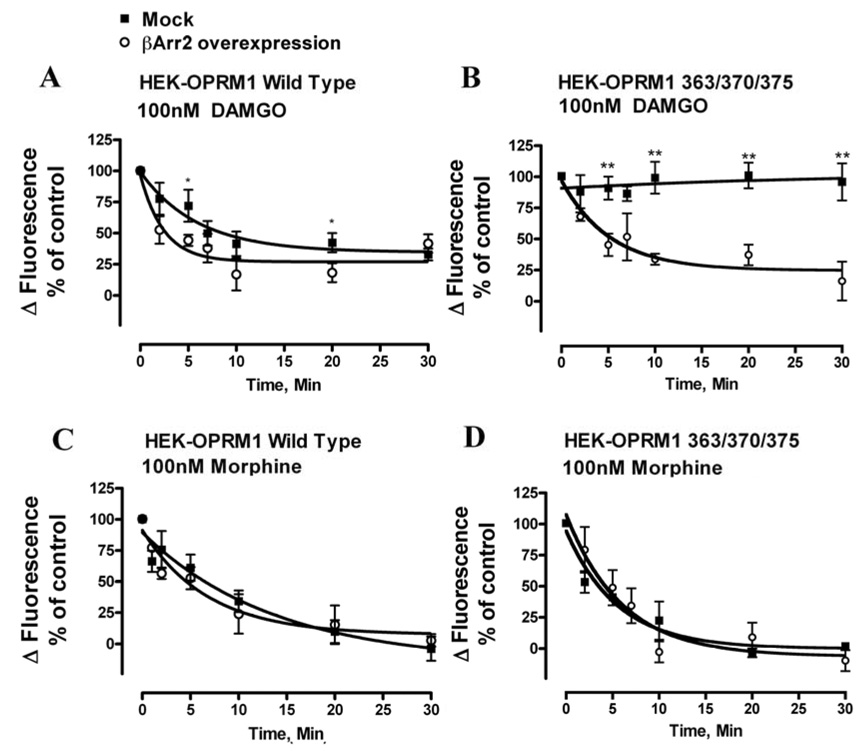
Although receptor phosphorylation is not required for morphine-induced receptor desensitization, βArr could still be the critical factor in OPRM1 desensitization due to the binding of βArr to GPCR independent of receptor phosphorylation. Thus, the role of βArr was examined firstly by the over-expression of the βArr-FLAG in HEK293 cells. As shown in Fig. 6A, over-expression of βArr2-FLAG increased the rate but not the extent of 100nM DAMGO-induced OPRM1 desensitization in HEK293 cells with wild type OPRM1. However, over-expression of βArr2-FLAG in HEK293 cells expressing the phosphorylation deficient OPRM1(363/370/375) increased both the extent and the rate of 100nM DAMGO-induced OPRM1 desensitization (Fig. 6B). As a matter of fact, when βArr2-FLAG was over-expressed, DAMGO pretreatment reduced receptor activity to the same extent in HEK293 cells expressing either wild type or OPRM1(363/370/375) (Fig. 6A and B). Such data indicated that over-expression of βArr2-FLAG could overcome the effect of phosphorylation deficient mutant on desensitization, which was consistent with other reports indicating GPCR phosphorylation increased the receptor affinity for βArr [6]. In contrast, 100nM morphine induced receptor desensitization was not affected by the over-expression of βArr2-FLAG in HEK293 cells expressing with either wild type or phosphorylation deficient OPRM1. (Fig. 6C and D). The rate and the extent of morphine-induced receptor desensitization were similar in cells with endogenous level or over-expressed level of βArr2.
Fig. 6. Effect of βArr2 overexpression on DAMGO- and morphine-induced OPRM1 desensitization in HEK293 cells.
The abilities of DAMGO to induce the desensitization of wild type OPRM1 and phosphorylation deficient OPRM1(363/370/375) were examined in HEK293 cells transfected with βArr2-FLAG (○) or with mock transfection (■). Cells were pretreated with 100nM DAMGO or morphine for the indicated time. The agonist-induced desensitization rate was calculated as described in the legend of Fig. 2B. Data were showed as the averages of n≥3 experiments in which wild type OPRM1 was pretreated with 100nM DAMGO (panel A) and 100nM morphine (panel C) or OPRM1(363/370/375) was pretreated 100nM DAMGO (panel B) and 100nM morphine (panel D) respectively. * denotes p < 0.05; and ** denotes p< 0.01.
To further demonstrate the role of βArr on morphine- and DAMGO-induced OPRM1 desensitization, mouse embryonic fibroblasts (MEF) cells isolated from wild type, βArr2 null mice (βArr2−/−) or βArr1 and βArr2 null mice (βArr1/2−/−) were used. These MEF cells were infected with adenovirus containing OPRM1 (Ad-OPRM1) to produce similar receptor level expressed as HEK-OPRM1 cells that was determined by receptor binding assay (Table 2). In these MEF cells, ADP did not evoke [Ca2+]i release, but another broad spectrum purinergic receptor agonist, ATP, evoked the [Ca2+]i release response. Similar to the observation in HEK293 cells, DAMGO and morphine potentiated ATP-induced [Ca2+]i release in MEF cells. Consistent with the HEK293 cells observations, morphine exhibited a partial agonist property in regulating the [Ca2+]i release. Similar partial agonist properties of morphine as compared to DAMGO were observed in all three types of MEF cells (Table 2).
Table 2. EC50 of morphine and DAMGO for OPRM1 expressed in various MEF cells.
Wild type, βArr2−/− and βArr1/2−/− MEF cells were infected with adenovirus containing OPRM1. Morphine- and DAMGO-induced [Ca2+]i release concentration-reponse curves were determined as described in Fig 1B. EC50 values were calculated by log(agonist)vs. response formula using the data analysis program GraphPad Prism. Radioligand binding experments were performed as described in material and methods. Data were showed as the averages of n≥3 experiments.
| MEF | Response | Receptor desensity, (pmol/mg) | |
|---|---|---|---|
| EC50, Morphine, (nM) | EC50 DAMGO , (nM) | ||
| WT | 138 ± 48 | 30.5 ± 1.4 | 0.37 ± 0.08 |
| βArr2−/− | 194 ± 50 | 68.3 ± 2.2 | 0.34 ± 0.11 |
| βArr1/2−/− | 61 ± 5 | 25.3 ± 3.2 | 0.75 ± 0.23 |
means ± SEM (n ≥3)
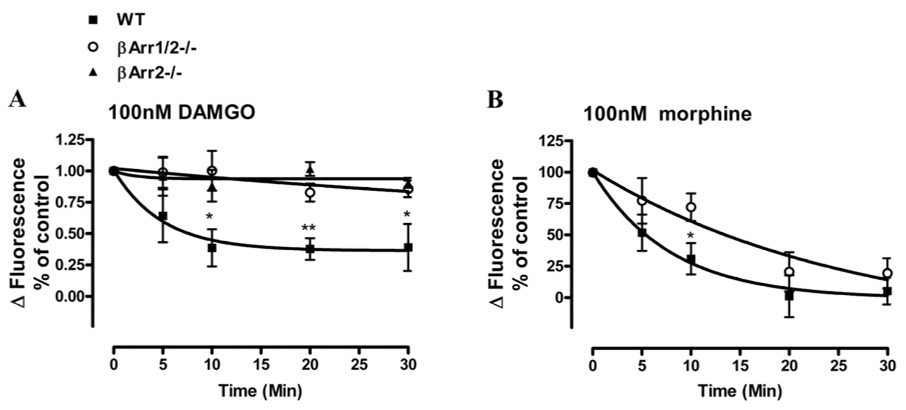
After the MEF cells were pretreated with these two OPRM1 agonists, both 100nM DAMGO and morphine reduced OPRM1 activity in wild type MEF cells. As shown in Figure 7, after 30 min pretreatment, 100nM morphine- and DAMGO-induced OPRM1 activities were 39±18% and 5±11% of control respectively. However, in βArr1/2−/− cells, after 30 min of 100nM DAMGO pretreatment, 1µM DAMGO-induced [Ca2+]i release was similar to those observed in the MEF cells not treated by the agonist. This observation supports the phosphorylation deficient mutants studies indicating that βArrs is critical in DAMGO-induced OPRM1 desensitization. Most likely βArr2 is the βArr subtype participated in DAMGO-induced OPRM1 desensitization. For in βArr2−/− MEF cells, DAMGO-induced receptor desensitization was attenuated to the same extent as that observed in βArr1/2−/− MEF cells. In these βArr null MEF cells, 91±4% of OPRM1 activity remained after 30 min 100nM DAMGO pretreatment (Fig. 7A). On the other hand, the presence of βArr is not a prerequisite for morphine-induced receptor desensitization. In contrast to DAMGO-induced receptor desensitization, 30 mins of 100 nM morphine pretreatment significantly reduced OPRM1 activity by 80±11% in the βArr1/2−/− MEF cells. Although the desensitization rate appeared to be slower when compared to that observed in WT MEF cells, the magnitude of morphine-induced OPRM1 desensitization remained similar (Fig. 7B). These data suggest that morphine-induced OPRM1 desensitization is βArr-independent, whereas DAMGO-induced OPRM1 desensitization is absolutely dependent on βArrs.
Fig. 7. Effect of βArr2 depletion on DAMGO- and morphine-induced OPRM1 desensitization in MEF cells.
Wild type, βArr2−/− and βArr1/2−/− MEF cells were infected with adenovirus containing OPRM1 and OPRM1 expressing level was monitored by receptor binding assay as described in experiment procedures. The abilities of 100nM DAMGO and morphine to induce the desensitization of OPRM1 in wild type MEF cells (■) βArr1/2−/− MEF cells (○) and βArr2−/− MEF cells (▲) was examined. Cells were pretreated with 100nM DAMGO or morphine, and agonist-induced desensitization was obtained as described in legend of Figure 2. Data summarize the average of n≥3 experiments in which the HEK293 cells were treated with 100nM DAMGO (panel A) and 100nM morphine (panel B) for various time to induce OPRM1 desensitization. *denotes p < 0.05 and ** denotes p< 0.01.
Discussion
GRK-mediated phosphorylation and subsequent βArrs recruitment are recognized as critical factors in receptor homologous desensitization [33, 34]. However the discrepancy between the kinetics of OPRM1 desensitization and receptor phosphorylation indicated the uncertainties in the OPRM1 desensitization mechanism. Although, such discrepancy was normally attributed to the relatively high level of OPRM1 in the heterologous expression system as illustrated when OPRM1 level was altered either by alkylating with β-furnaltrexamine (βFNA) or by using an ecdysone-inducible mammalian expression system [35], the methods used to monitor the desensitization was another possible reason [36]. Because the relatively high efficiency of OPRM1 to inhibit adenylyl cyclase activity through Gα resulted in the “spare receptors” (response is not directly correlated to receptor occupancy adenylyl cyclase activity might not be a sensitive enough monitor for rapid desensitization. In contrast, the intracellular calcium release mediated by the less efficient signal transducer Gβγ should be a better choice [23, 24]. By measuring the Gβγ-mediated [Ca2+]i release, we demonstrate a rapid loss of OPRM1 responsiveness within minutes after morphine or DAMGO treatment. Furthermore, this time course of receptor desensitization correlated to the rate of receptor phosphorylation and βArrs recruitment that were reported previously [8, 17].
By taking advantage of this sensitive indicator of OPRM1 activity, we were able to study in details the OPRM1 desensitization mechanism. Further experiments on the phosphorylation deficient OPRM1 and βArr1/2−/− MEF cells suggested the existence of multiple mechanisms on OPRM1 desensitization. On the one hand, DAMGO-induced OPRM1 desensitization strictly follows the classic GPCR desensitization mechanism; i.e. it is regulated by receptor phosphorylation and required βArr. Mutation of the putative GRK-mediated phosphorylation sites and alteration of intracellular βArr expression level significantly altered DAMGO-induced OPRM1 desensitization. On the other hand, although similar to DAMGO, pretreatment of morphine caused the rapid loss of receptor responsiveness, it was shown clearly from our current studies that the morphine-induced desensitization was receptor phosphorylation- and βArr-independent. Furthermore, by over-expressing βArr or completely depleting βArr, it is the first time that βArr-independent OPRM1 rapid desensitization was shown.
Previously, in vitro OPRM1 desensitization was used as molecular mechanism to explain the tolerance development in vivo [33]. Based on the observations with βArr recruitment and receptor phosphorylation, two theories have been proposed. One theory suggested in vitro OPRM1 desensitization is the basis for in vivo tolerance. Thus, the degree of in vivo tolerance will reflect the amount of receptor being desensitized. This theory is supported by the observation with the βArr2−/− mice, in which morphine-mediated analgesia tolerance was completely attenuated as monitored by hot-plate test [1]. However, the ability of morphine to induce antinociceptive tolerance as measured by tail-flick tests in the βArr2−/− mice [37], and the inability of morphine-activated OPRM1 to recruit βArr indicated factors other than βArr2 participate in morphine-induced tolerance. Our current observed morphine-mediated βArr-independent desensitization suggests alternative pathways are involved in the in vitro morphine-induced desensitization, and may influence morphine-mediated in vivo tolerance development.
The other theory considered βArr-mediated receptor internalization is the pathway opioid receptor utilized for resensitization during the receptor recycling process, which can prevent the further loss of OPRM1 responsiveness [38, 39]. Severe tolerance development to morphine is due to the agonist’s inability to induce receptor internalization and subsequent resensitization. Such theory appears to be supported by our current findings. Since DAMGO is able to induce OPRM1 internalization while morphine cannot, the higher extent of desensitization after 30 min of morphine treatment than that of DAMGO treatment could reflect receptor resensitization might be involved in the overall desensitization process.
Normally receptor phosphorylation was suggested to be mediated by GRK and such phosphorylation increase the affinity of agonist-receptor complex for βArr. GRKs overexpression was the most commonly used method to study the effect of phosphorylation on receptor desensitization[33, 40]. In order to overcome the difficulties in correlating the phosphorylation states of the receptor and desensitization, previous report has investigated the effect of receptor phosphorylation on desensitization by eliminating the putative phosphorylation sites in OPRM1 [41]. However, involvement of receptor phosphorylation in rapid desensitization was still unclear, since the relatively long agonist pretreated time and high agonist concentration resulted in OPRM1 desensitization rate reflecting both the uncoupling of OPRM1 from the G-protein and the internalization of OPRM1. Our current agonist treatment paradigm, <30 min, should minimize the roles of receptor internalization and resensitization in the receptor desensitization process. Clearly, phosphorylation of Ser375 participated in the DAMGO-induced desensitization process. Mutation of the Ser375 to Ala but not the other putative phosphorylation residues blunted the DAMGO-induced receptor desensitization. Our current findings are consistent with previous study that indicated Ser375 is the major phosphorylation residue that regulates DAMGO-induced OPRM1 internalization[27]. Although morphine also induced Ser375 phosphorylation [32], the βArr-dependent receptor internalization was not induced by morphine unless either GRK2 or βArr was over-expressed in the system[3, 16]. Furthermore, our current studies indicated that phosphorylation-dependent desensitization was not observed when morphine was used in current study. Morphine-induced OPRM1 desensitization was not affected by the over-expression of βArr, was present in the βArr1/2−/− MEF cells, and was similar in wild type and phosphorylation minus mutants. This is in contrast to the ability of over-expression of βArr to promote morphine-induced receptor internalization [16].
If morphine-induced OPRM1 desensitization does not involve GRK-mediated receptor phosphorylation and subsequent βArr recruitment, then what are the possible mechanisms for such agonist-induced receptor desensitization? Several protein kinases such as PKC [40], PKA [42], and ERK[43] have been suggested to be involved in OPRM1 desensitization. After morphine treatment, increases in PKC or ERK activities have been reported [44, 45]. Although only chronic but not acute treatment of morphine could up-regulate PKA activity [46], all three protein kinases have been shown to phosphorylate OPRM1, thus resulting in receptor desensitization [17, 47–49]. Recently, Src activation has also been recognized to be involved in OPRM1 signal regulation [50]. Although the direct phosphorylation of OPRM1 by the Src kinase has not been reported, the Tyr phosphorylation of OPRM1 [51] was observed. In addition, OPRD1 has been shown to be Src kinase substrate [52, 53]. Beside directly phosphorylating receptor, these protein kinases were shown to phosphorylate G-protein subunits or OPRM1 signal components, which could eventually lead to the desensitization OPRM1 signals[54–57]. Whether either one or all of these protein kinases is involved in the rapid desensitization of OPRM1-mediated [Ca2+]i release needs to be investigated further.
Our current studies demonstrate the agonist-dependent desensitization exists, which is consistent with the previous studies on DAMGO- and morphine-induced potassium current desensitization[40]. Moreover, our studies clearly demonstrated that morphine could induce receptor desensitization in the absence of receptor phosphorylation and βArr. Since published observations have supported the role of βArr2 in morphine-induced tolerance development [1], our current observations might simply reflect the situation with the [Ca2+]i release. However, depletion of GRK3 in vivo only attenuate fentanyl- but not morphine-induced tolerance [14], and the ability of PKC and PKA inhibitors [37, 58–60] or the absence of PKCγ or PKCε to blunt morphine-induced tolerance development in vivo [61, 62] suggested agonist-dependent mechanism on opioid tolerance development. Such in vivo results are consistent to our observation in current study, thus apparent difference exists between DAMGO- and morphine-induced OPRM1 desensitization. Such agonist-dependent desensitization mechanism might contribute to the differences in these agonists’ effects in vivo.
Conclusion
In summary, Gβγ-mediated [Ca2+]i release provided a sensitive method to study the OPRM1 desensitization mechanism in details. This is the first time we clearly demonstrated βArr was absolutely required in DAMGO-induced OPRM1 desensitization. In addition, agonist-induced OPRM1 phosphorylation on each individual site did not function equally in DAMGO-induced OPRM1 desensitization process. Current studies also indicated that OPRM1 desensitization mechanism was agonist-dependent. Thus morphine-induced OPRM1 desensitization was not affected by receptor phosphorylation and the absence of βArr. This apparent difference correlates to agonist function in vivo, and may contribute to morphine-induced OPRM1 tolerance.
Supplementary Material
Morphine-mediated [Ca2+]i release required P2Y receptor co-activation. Data shows the real time intracellular fluorescence change in raw fluorescence unit (RFU). HEK293 cells were cultured as described in materials and methods. Fluorescence dye to detect free [Ca2+]i was added 1 hour before compound injection. After 30 second baseline reading, as indicated by the arrow, HEK293 cells expressing OPRM1 were injected with 200nM ADP ( ), 1µM morphine (□), 200nM ADP with 1µM morphine (
), 1µM morphine (□), 200nM ADP with 1µM morphine ( ), 200nM ADP, 1µM morphine and 30µM Naloxone (
), 200nM ADP, 1µM morphine and 30µM Naloxone ( ) respectively.
) respectively.
Acknowledgement
This research was supported in parts by National Institutes of Healthy grants DA007339, DA016674, DA000564 and DA011806. H.H.L. and P.Y.L. are recipients of K05-DA70544 and K05-DA00513, respectively. Dr. Robert Lefkowitz (Duke University, NC) generously provided the the wild type, βArr2−/− and βArr1/2−/− Mouse Embryonic Fibroblasts cells used in current studies. Dr. Mario Ascoli (University of Iowa, Iowa City) generously provided the βArr2FLAG construct.
Abbreviations
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- OPRM1
μ-opioid receptor
- βArr
β-arrestin
- OPRD1
δ-opioid receptor
- [Ca2+]i
intracellular Ca2+
- P2Y receptor
purinergic receptor
- DAMGO
[D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- MEF cell
mouse embryonic fibroblasts
- HEK293 cell
human embryonic kidney cell
- βFNA
β-furnaltrexamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Nature. 2000;408(6813):720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 2.Finn AK, Whistler JL. Neuron. 2001;32(5):829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Proc Natl Acad Sci U S A. 1998;95(12):7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Nature. 1996;383(6603):819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 5.Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Proc Natl Acad Sci U S A. 1997;94(4):1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefkowitz RJ. J Biol Chem. 1998;273(30):18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 7.Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, Hosey MM, Benovic JL. J Biol Chem. 1995;270(2):720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- 8.Barak LS, Ferguson SS, Zhang J, Caron MG. J Biol Chem. 1997;272(44):27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- 9.Roth A, Kreienkamp HJ, Meyerhof W, Richter D. J Biol Chem. 1997;272(38):23769–23774. doi: 10.1074/jbc.272.38.23769. [DOI] [PubMed] [Google Scholar]
- 10.Palmer TM, Benovic JL, Stiles GL. J Biol Chem. 1996;271(25):15272–15278. doi: 10.1074/jbc.271.25.15272. [DOI] [PubMed] [Google Scholar]
- 11.Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV. J Biol Chem. 1999;274(11):6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- 12.Hasbi A, Polastron J, Allouche S, Stanasila L, Massotte D, Jauzac P. J Neurochem. 1998;70(5):2129–2138. doi: 10.1046/j.1471-4159.1998.70052129.x. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Wehmeyer A, Schulz K. J Pharmacol Exp Ther. 2002;300(2):376–384. doi: 10.1124/jpet.300.2.376. [DOI] [PubMed] [Google Scholar]
- 14.Terman GW, Jin W, Cheong YP, Lowe J, Caron MG, Lefkowitz RJ, Chavkin C. Br J Pharmacol. 2004;141(1):55–64. doi: 10.1038/sj.bjp.0705595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. J Neurochem. 1995;65(4):1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- 16.Whistler JL, von Zastrow M. Proc Natl Acad Sci U S A. 1998;95(17):9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Kouhen R, Kouhen OM, Law PY, Loh HH. J Biol Chem. 1999;274(14):9207–9215. doi: 10.1074/jbc.274.14.9207. [DOI] [PubMed] [Google Scholar]
- 18.Kovoor A, Nappey V, Kieffer BL, Chavkin C. J Biol Chem. 1997;272(44):27605–27611. doi: 10.1074/jbc.272.44.27605. [DOI] [PubMed] [Google Scholar]
- 19.Celver JP, Lowe J, Kovoor A, Gurevich VV, Chavkin C. J Biol Chem. 2001;276(7):4894–4900. doi: 10.1074/jbc.M007437200. [DOI] [PubMed] [Google Scholar]
- 20.Lowe JD, Celver JP, Gurevich VV, Chavkin C. J Biol Chem. 2002;277(18):15729–15735. doi: 10.1074/jbc.M200612200. [DOI] [PubMed] [Google Scholar]
- 21.Prather PL, Song L, Piros ET, Law PY, Hales TG. J Pharmacol Exp Ther. 2000;295(2):552–562. [PubMed] [Google Scholar]
- 22.Dang VC, Williams JT. Mol Pharmacol. 2005;68(4):1127–1132. doi: 10.1124/mol.105.013185. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SH, Lo TM, Loh HH, Thayer SA. Mol Pharmacol. 1999;56(5):902–908. doi: 10.1124/mol.56.5.902. [DOI] [PubMed] [Google Scholar]
- 24.Jin W, Lee NM, Loh HH, Thayer SA. J Neurosci. 1994;14(4):1920–1929. doi: 10.1523/JNEUROSCI.14-04-01920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okajima F, Tomura H, Kondo Y. Biochem J. 1993;290(Pt 1):241–247. doi: 10.1042/bj2900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor M, Henderson G. Br J Pharmacol. 1996;117(2):333–340. doi: 10.1111/j.1476-5381.1996.tb15195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Kouhen R, Burd AL, Erickson-Herbrandson LJ, Chang CY, Law PY, Loh HH. J Biol Chem. 2001;276(16):12774–12780. doi: 10.1074/jbc.M009571200. [DOI] [PubMed] [Google Scholar]
- 28.Yeo A, Samways DS, Fowler CE, Gunn-Moore F, Henderson GJ. Neurochem. 2001;76(6):1688–1700. doi: 10.1046/j.1471-4159.2001.00185.x. [DOI] [PubMed] [Google Scholar]
- 29.Samways DS, Li WH, Conway SJ, Holmes AB, Bootman MD, Henderson G. Biochem. J. 2003;375:713–720. doi: 10.1042/BJ20030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovoor A, Celver JP, Wu A, Chavkin C. Mol Pharmacol. 1998;54(4):704–711. [PubMed] [Google Scholar]
- 31.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Proc Natl Acad Sci U S A. 1990;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V. Embo J. 2004;23(16):3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connor M, Osborne PB, Christie MJ. Br J Pharmacol. 2004;143(6):685–696. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luttrell LM, Lefkowitz RJ. J Cell Sci. 2002;115(Pt 3):455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 35.Law PY, Erickson LJ, El-Kouhen R, Dicker L, Solberg J, Wang W, Miller E, Burd AL, Loh HH. Mol Pharmacol. 2000;58(2):388–398. doi: 10.1124/mol.58.2.388. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. J Neurosci. 2002;22(13):5769–5776. doi: 10.1523/JNEUROSCI.22-13-05769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohn LM, Lefkowitz RJ, Caron MG. J Neurosci. 2002;22(23):10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Neuron. 1999;23(4):737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 39.He L, Fong J, von Zastrow M, Whistler JL. Cell. 2002;108(2):271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 40.Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Mol Pharmacol. 2006;70(2):676–685. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- 41.Qiu Y, Law PY, Loh HH. J Biol Chem. 2003;278(38):36733–36739. doi: 10.1074/jbc.M305857200. [DOI] [PubMed] [Google Scholar]
- 42.Pitcher J, Lohse MJ, Codina J, Caron MG, Lefkowitz RJ. Biochemistry. 1992;31(12):3193–3197. doi: 10.1021/bi00127a021. [DOI] [PubMed] [Google Scholar]
- 43.Polakiewicz RD, Schieferl SM, Dorner LF, Kansra V, Comb MJ. J Biol Chem. 1998;273(20):12402–12406. doi: 10.1074/jbc.273.20.12402. [DOI] [PubMed] [Google Scholar]
- 44.Narita M, Makimura M, Feng Y, Hoskins B, Ho IK. Brain Res. 1994;650(1):175–179. doi: 10.1016/0006-8993(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 45.Law PY, Wong YH, Loh HH. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 46.Nestler EJ, Tallman JF. Mol Pharmacol. 1988;33(2):127–132. [PubMed] [Google Scholar]
- 47.Bernstein MA, Welch SP. Brain Res Mol Brain Res. 1998;55(2):237–242. doi: 10.1016/s0169-328x(98)00005-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Yu Y, Mackin S, Weight FF, Uhl GR, Wang JB. J Biol Chem. 1996;271(19):11449–11454. doi: 10.1074/jbc.271.19.11449. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt H, Schulz S, Klutzny M, Koch T, Handel M, Hollt V. J Neurochem. 2000;74(1):414–422. doi: 10.1046/j.1471-4159.2000.0740414.x. [DOI] [PubMed] [Google Scholar]
- 50.Walwyn W, Evans CJ, Hales TG. J Neurosci. 2007;27(19):5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLaughlin JP, Chavkin C. Mol Pharmacol. 2001;59(6):1360–1368. doi: 10.1124/mol.59.6.1360. [DOI] [PubMed] [Google Scholar]
- 52.Kramer HK, Andria ML, Kushner SA, Esposito DH, Hiller JM, Simon EJ. Brain Res Mol Brain Res. 2000;79(1–2):55–66. doi: 10.1016/s0169-328x(00)00097-8. [DOI] [PubMed] [Google Scholar]
- 53.Kramer HK, Andria ML, Esposito DH, Simon EJ. Biochem Pharmacol. 2000;60(6):781–792. doi: 10.1016/s0006-2952(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 54.Chakrabarti S, Gintzler AR. Mol Pharmacol. 2007;72(3):753–760. doi: 10.1124/mol.107.036145. [DOI] [PubMed] [Google Scholar]
- 55.Strassheim D, Law PY, Loh HH. Mol Pharmacol. 1998;53(6):1047–1053. [PubMed] [Google Scholar]
- 56.Strassheim D, Malbon CC. J Biol Chem. 1994;269(19):14307–14313. [PubMed] [Google Scholar]
- 57.Yasuda H, Lindorfer MA, Myung CS, Garrison JC. J Biol Chem. 1998;273(34):21958–21965. doi: 10.1074/jbc.273.34.21958. [DOI] [PubMed] [Google Scholar]
- 58.Smith FL, Javed RR, Smith PA, Dewey WL, Gabra BH. Pharmacol Res. 2006;54(6):474–480. doi: 10.1016/j.phrs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. Pain. 2007;127(1–2):129–139. doi: 10.1016/j.pain.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Javed RR, Dewey WL, Smith PA, Smith FL. Eur J Pharmacol. 2004;492(2–3):149–157. doi: 10.1016/j.ejphar.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 61.Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. Pain. 2001;94(3):245–253. doi: 10.1016/S0304-3959(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 62.Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ, Roberts AJ, Hodge CW, Messing RO. Genes Brain Behav. 2007;6(4):329–338. doi: 10.1111/j.1601-183X.2006.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morphine-mediated [Ca2+]i release required P2Y receptor co-activation. Data shows the real time intracellular fluorescence change in raw fluorescence unit (RFU). HEK293 cells were cultured as described in materials and methods. Fluorescence dye to detect free [Ca2+]i was added 1 hour before compound injection. After 30 second baseline reading, as indicated by the arrow, HEK293 cells expressing OPRM1 were injected with 200nM ADP ( ), 1µM morphine (□), 200nM ADP with 1µM morphine (
), 1µM morphine (□), 200nM ADP with 1µM morphine ( ), 200nM ADP, 1µM morphine and 30µM Naloxone (
), 200nM ADP, 1µM morphine and 30µM Naloxone ( ) respectively.
) respectively.