Abstract
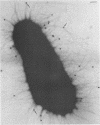
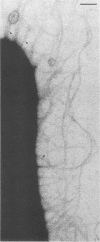
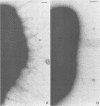
Salivary proline-rich proteins (PRPs), which were purified from parotid saliva, were adsorbed onto 15-nm-diameter gold particles to visualize specific binding of the salivary molecules to Actinomyces viscosus type 1 fimbriae. Negatively stained preparations incubated with PRP-gold conjugates but not bovine serum albumin-gold complexes bound specifically to bacteria possessing type 1 fimbriae, A. viscosus T14V-J1 and 5519. Binding of the PRP-gold probes to strains deficient in type 1 fimbriae, i.e., strains 5951 (type 2 fimbriae only) and 147 (no fimbriae), was negligible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan M. J., Cisar J. O., Vatter A. E., Sandberg A. L. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect Immun. 1984 Nov;46(2):459–464. doi: 10.1128/iai.46.2.459-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., Clark W. B., Curl S. H., Hurst-Calderone S., Sandberg A. L. Mutants of Actinomyces viscosus T14V lacking type 1, type 2, or both types of fimbriae. Infect Immun. 1988 Nov;56(11):2984–2989. doi: 10.1128/iai.56.11.2984-2989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Beem J. E., Nesbitt W. E., Cisar J. O., Tseng C. C., Levine M. J. Pellicle receptors for Actinomyces viscosus type 1 fimbriae in vitro. Infect Immun. 1989 Oct;57(10):3003–3008. doi: 10.1128/iai.57.10.3003-3008.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Cisar J. O. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect Immun. 1984 Feb;43(2):497–501. doi: 10.1128/iai.43.2.497-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Buivids I. A., Simardone J. R. Actinomyces viscosus fibril antigens detected by immunogold electron microscopy. Infect Immun. 1989 Apr;57(4):1327–1331. doi: 10.1128/iai.57.4.1327-1331.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan W. D., Ackerman G. A. Adsorption of horseradish peroxidase, ovomucoid and anti-immunoglobulin to colloidal gold for the indirect detection of concanavalin A, wheat germ agglutinin and goat anti-human immunoglobulin G on cell surfaces at the electron microscopic level: a new method, theory and application. J Histochem Cytochem. 1977 Nov;25(11):1187–1200. doi: 10.1177/25.11.21217. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Cisar J. O., Clark W. B. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect Immun. 1988 Nov;56(11):2990–2993. doi: 10.1128/iai.56.11.2990-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988 Feb;56(2):439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. L., Hodges G. M., Trejdosiewicz L. K., Livingston D. C. Colloidal gold markers and probes for routine application in microscopy. J Microsc. 1981 Aug;123(Pt 2):201–213. doi: 10.1111/j.1365-2818.1981.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Bennick A., Schlesinger D. H., Minaguchi K., Madapallimattam G., Schluckebier S. K. The primary structures of six human salivary acidic proline-rich proteins (PRP-1, PRP-2, PRP-3, PRP-4, PIF-s and PIF-f). Biochem J. 1988 Oct 1;255(1):15–21. doi: 10.1042/bj2550015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I., Franzblau C. Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry. 1971 Nov;10(23):4233–4238. doi: 10.1021/bi00799a013. [DOI] [PubMed] [Google Scholar]
- Robinson E. N., Jr, Clemens C. M., McGee Z. A., Cannon J. G. Immunoelectron microscopic localization of outer membrane proteins II on the surface of Neisseria gonorrhoeae. Infect Immun. 1988 Apr;56(4):1003–1006. doi: 10.1128/iai.56.4.1003-1006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]