Abstract
The marine mollusk Aplysia is a useful model organism for studying the cellular bases of behavior and plasticity. However, molecular studies of Aplysia have been limited by the lack of genomic information. Recently, a large scale characterization of neuronal transcripts was performed in A. californica. Here, we report the analysis of a parallel set of neuronal transcripts from a closely related species A. kurodai found in the northwestern Pacific. We collected 4,859 nonredundant sequences from the nervous system tissue of A. kurodai. By performing microarray and real-time PCR analyses, we found that ApC/EBP, matrilin, antistasin, and eIF3e clones were significantly up-regulated and a BAT1 homologous clone was significantly down-regulated by 5-HT treatment. Among these, we further demonstrated that the Ap-eIF3e plays a key role in 5-HT-induced long-term facilitation (LTF) as a positive regulator.
Keywords: expressed sequence tag, long-term facilitation
The marine mollusk Aplysia is an important model organism for studying the cellular and molecular mechanisms underlying learning and memory (1–3). There are four major advantages of using Aplysia for the studies of synaptic plasticity, learning, and memory storage. First, Aplysia is capable of a variety of interesting behaviors that can be modified by various forms of learning to give rise to both short- and long-term memories. Second, Aplysia has a relatively simple nervous system, consisting of only ≈2 × 104 central nerve cells compared with the 1011 neurons of mammalian brains. Third, the giant neurons of Aplysia are among the largest somatic cell types in the animal kingdom, enabling one to conduct various genetic manipulations, such as the microinjection of plasmid, RNA, or protein on the level of a single neuron (4). Finally, many neurons are uniquely identifiable in every animal of the species and can be related to the animal's behaviors (5, 6). Because of these advantages, the cell and molecular biology of certain key neural circuits in the nervous system of Aplysia have been studied extensively.
However, molecular level analyses in Aplysia have been limited until recently by the lack of genomic information. As a first step, Moccia et al. characterized the gene expression profiles of the processes of Aplysia sensory neurons, thereby demonstrating the usefulness of high-throughput gene analysis in studying synaptic plasticity (7). Next, the whole mitochondrial genome of A. californica was sequenced (8) and a large scale expressed sequence tag (EST) analysis for this species was completed (9). Moroz, Kandel, and colleagues sequenced more than 200,000 ESTs, which represented over 65,000 nonredundant sequences from A. californica cDNA libraries derived from several sources including the whole CNS, individual ganglia, identified neurons, and identified processes of identified neurons. Of those putative unique transcripts, they were able to annotate approximately 4,900 genes with clearly recognized orthologs in other organisms (9). Further characterization of neuronal transcripts in Aplysia requires both functional and comparative studies. As a first step in that direction, we have obtained complementary data from A. kurodai, a closely related species living in the northwestern Pacific. Both organisms represent a relatively recent speciation event and the topological organization of the central ganglia is nearly identical between two species in which the majority of individual neurons are clearly identifiable.
Here, we describe and annotate the collection of 11,493 ESTs generated from the central nervous system of A. kurodai. Using this collection, we constructed custom microarrays composed of approximately 7,000 neuronal cDNA clones. These arrays were used to identify genes differentially expressed in response to 5-HT known as a key neurotransmitter for LTF in Aplysia (10). Finally, we characterized a gene, Aplysia eukaryotic translation initiation factor 3 (Ap-eIF3e), as a molecular switch that converts short-term facilitation (STF) to LTF.
Results
Generation and Assembly of Aplysia kurodai ESTs.
By sequencing cDNA libraries from the CNS of A. kurodai, we constructed a representative EST database that facilitates the mining of genes related to basal neuronal functions and plasticity. Sequence analyses of the previously cloned Aplysia genes showed that many transcripts contained relatively long UTRs in their 3′ ends (11, 12) and might cause problems in the initial annotation of protein-coding genes. Therefore, we generated bidirectional CNS cDNA libraries using an oligo-capping method that enabled us to obtain full-length and 5′-end-enriched cDNAs (13–15). To increase the complexity of cDNA clones, we made three additional libraries: a nondirectional random-primed CNS library, a 5-HT treated CNS library that is 3′ end-enriched, and a buccal muscle library. A total of 11,755 sequences was obtained, trimmed of vector and low-quality sequences, yielding 11,493 ESTs that measured an average 595 bp (see supporting information (SI) Table S1).
These 11,493 high-quality ESTs were assembled and clustered by using the nonsupervised clustering method, assuming that the clustered ESTs originated from the same gene. Part (32.8%) of the original ESTs (3,765) was composed of singletons (or occurred only once in the collection). The remaining 7,728 clones fell into 996 clusters with sizes ranging from two (515 clusters) to 2,274 (one cluster), while 57.3% of the total ESTs belonged to cluster sets of 10 or fewer. The clustered ESTs were assembled by using the CAP3 assembly program (16), resulting in 4,859 nonredundant transcripts (1,094 contigs and 3,765 singletons), ranging up to 3,200 bp in length (Table 1).
Table 1.
Aplysia kurodai contig statistics
| Total sequences analyzed | 11493 |
| Number of ESTs in contigs | 7728 |
| Number of contigs | 1094 |
| Number of singletons | 3765 |
| Number of putative transcripts (assembled sequences) | 4859 |
| Number of contigs in size of | |
| < 1 kb | 780 |
| 1 kb ≤ size < 2 kb | 286 |
| 2 kb ≤ size < 3 kb | 27 |
| 3 kb ≤ size < 4 kb | 2 |
| Average contig size | 949 bp |
Of the total assembled putative transcripts, 1,648 sequences matched proteins from the GenBank nonredundant database at a BLASTX e-value cutoff of 10−5. These sequences were further classified by using gene ontology (GO) categories (17) as described in Tables S2 and S3. Original ESTs were submitted to GenBank under accession numbers EY392795–EY396002 and EY416138–EY424374; all analyzed data and detailed information are available through a website (http://seahare.org).
Comparison of Neuronal Transcripts Between Two Aplysia Species.
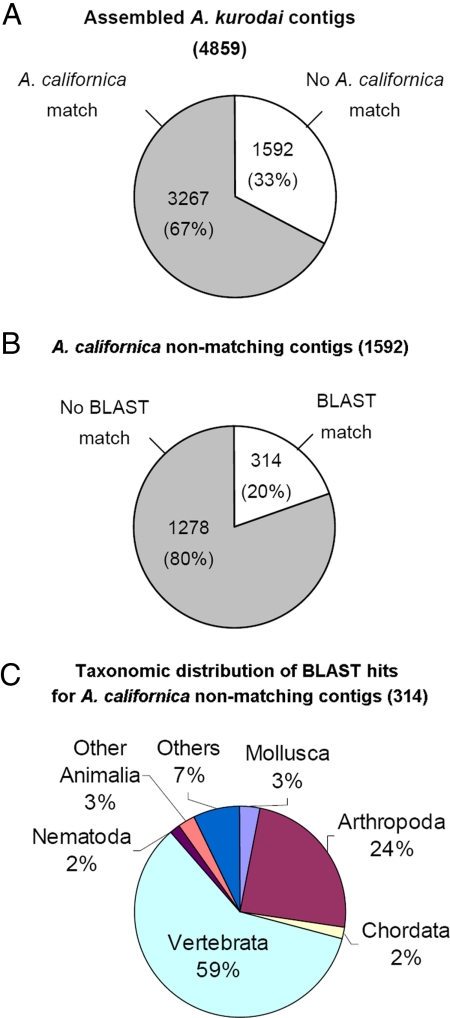
First, we mapped A. kurodai contigs to 65,055 putative A. californica nonredundant contigs so as to identify orthologous sequences. Of the 4,859 A. kurodai contigs, 3,267 (67%) were matched to known A. californica sequences and 1,592 (33%) had no significant matches (BLASTN; e ≤ 10−10) (Fig. 1). It is likely that many of these nonmatching sequences are fragments of nonoverlapping transcripts. Fig. 1B summarizes the proportion of these A. californica nonmatching A. kurodai sequences with and without BLASTX (18) matches in the nonredundant protein (nr) database (e ≤ 10−3) from National Center for Biotechnology Information. The majority (1,278 of 1,592; 80%) of sequences had no matches in public databases. Of the 314 BLAST-matching transcripts, only 3% were homologous to known protein sequences from Mollusca, including the genus Aplysia (Fig. 1C).
Fig. 1.
Comparative analysis of A. kurodai with A. californica. (A) The proportion of A. kurodai contigs with and without matches in A. californica EST set (BLASTN; e ≤ 10−10). (B) A. kurodai contigs without matches in A. californica EST were separately analyzed for BLASTX matches in nr protein database (BLASTX; e ≤ 10−3). (C) A. californica nonmatching-A. kurodai contigs with matches in nr database were classified by the homologous proteins and their species. Twenty-four percent of the sequences were homologous to the sequences from Arthropoda, including Drosophila and Apis. A large portion, 59% of BLASTX-hit genes, showed homology to sequences from a variety of species in Vertebrata, from Danio rerio (zebrafish) to Homo sapiens. Others were mostly related to proteins from Nematoda or other Animalia. It is interesting that some (7%) matched to bacterial (Mycobacterium, Bacillus, and Silicibacter) or viral (entomopoxvirus and bacteriophage) protein sequences. These sequences might result from the infection or contamination of Aplysia nervous system.
Differentially Expressed Genes in Response to 5-HT.
To monitor the changes in gene expression in response to 5-HT stimulation, we constructed A. kurodai cDNA microarrays. A total of 6,912 clones was successfully amplified by PCR and printed on glass slides. To investigate the changes of gene expression by 5-HT treatment, Aplysia were treated with 5-HT in vivo (250 μM, 2 h) (11), and pleural ganglia that contain sensory clusters were isolated. Amplified RNAs from the pleural ganglia of 5-HT treated or nontreated animals were used to generate a fluorescence-labeled probe and hybridized to the microarray (see details in the Methods section and/or SI). After filtering out low quality spots, 27 clones were found to be differentially expressed in 5-HT-treated pleural ganglia. (Table S4). Table 2 shows the list of selected annotated clones. The previously characterized immediate-early gene, ApC/EBP, was also found to be up-regulated after 5-HT treatment.
Table 2.
Real-time PCR confirmation about differentially expressed clones by >2-fold 2 h after 5-HT treatment in microarray
| EST name | BLASTX result* | GenBank accession number | Ratio of medians | Short description of real-time PCR results† |
|---|---|---|---|---|
| Up-regulated clones | ||||
| 5CAP092402_H04_760 | Matrilin (AAN61407, 1.00E-33) | EY418286 | 5.377 | Significant |
| 5CAP090501-pMES_D10_142 | Antistasin (P38977, 2.00E-25) | EY417467 | 4.830 | Significant |
| 5CAP031402_pMES_B12_1600 | Cathepsin L-like cysteine proteinase precursor (AAQ22984, 5.00E-05) | EY417083 | 4.419 | No change |
| 5CAP090504-pMES_E03_435 | alpha tubulin 2 (AAM09674, 6.00E-48) | EY417749 | 2.984 | No change |
| 5CAP092402_B10_694 | LOC443610 protein (AAI28922, 7.00E-55) | EY418226 | 2.838 | No change |
| ApC/EBP (positive control) | ApC/EBP (AAA18286, 0) | 2.594 | Significant | |
| 5HTCNS122105-T3-C10–628 | Eukaryotic translation initiation factor 3, subunit 6 (NP_001559, 5.00E-73) | EY416763 | 2.436 | Significant |
| 5CAP090501-pMES_F08_164 | Hypothetical protein (XP_001176996, 6.00E-33) | EY417489 | 2.230 | No change |
| Down-regulated clones | ||||
| 5CAP101001_F09_1029 | Kazal proteinase inhibitor (ABL74453, 2.00E-22) | EY418526 | 0.346 | No change |
| 5CAP120302-pMES_C06_1166 | unnamed protein product (CAG08644, 2.00E-14) | EY419713 | 0.356 | Reverse trend |
| 5CAP031405_pMES_G09_1891 | hCG1999844 (EAX04582, 0.013) | EY417337 | 0.460 | No change |
| 5CAP101001_H07_1051 | BAT1 homolog (AAQ13472, 2.00E-26) | EY418548 | 0.486 | Significant |
*BLASTX results are described as: gene description (accession number of BLASTX matched gene, E-value).
†Significant means P < 0.05 by student's t test; trend or reverse trend means 0.05 < P ≤ 0.10 by student's t test; no change means P > 0.10.
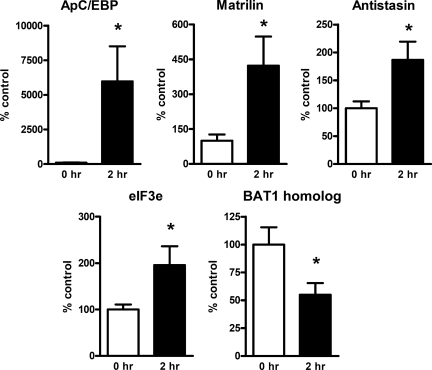
We also performed the qRT-PCR analysis to further validate predicted 5-HT regulated genes (Fig. 2). Based on BLASTX search results, 13 clones that have the matching proteins in the database were selected for qRT-PCR analysis. qRT-PCR confirmed that four clones (ApC/EBP, matrilin, antistasin, and eIF3e) were significantly up-regulated by 5-HT treatment, and one clone (BAT1 homolog) was significantly down-regulated by 5-HT treatment (Fig. 2). The expression of the remaining clones will be examined in a future study.
Fig. 2.
Real-time RT-PCR analyses on the selected 5-HT regulated genes. Four genes (ApC/EBP, matrilin, antistasin, and eIF3e) were found to be significantly increased, and one gene (BAT1 homolog) was significantly decreased in response to 5-HT treatment (P < 0.05, Student t test).
Role of Aplysia eIF3e in 5-HT-Induced, Long-Term Facilitation at the Sensorimotor Synapse.
As the next step, we tested whether a 5-HT-regulated gene, eIF3e/subunit 6 (Int6) (Table 2), contributes to the long-term plasticity. First, we obtained a full-length cDNA from A. kurodai nervous system (for the cloning information, see Materials and Methods). A cloned Ap-eIF3e/Int6 (GenBank accession number EU791459) showed 56–73% amino acid sequence identity in comparison with eIF3e/Int6 of other species (Fig. S1). The C-terminal PCI domain, which is involved in translation initiation and protein degradation, is also well conserved in Ap-eIF3e (19, 20).
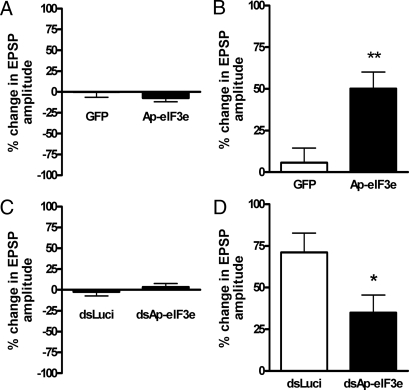
To examine the role of Ap-eIF3e in long–term facilitation, we overexpressed Ap-eIF3e in a sensory neuron of sensory to motor cocultured synapse. Overexpression of Ap-eIF3e itself did not affect the synaptic strength one day after microinjection (% change in EPSP amplitude, 92.7 ± 4.6, n = 17 and 100.0 ± 6.4, n = 14 for Ap-eIF3e overexpressing and control synapses, respectively; P = 0.351, Fig. 3A). One pulse of 5-HT treatment, however, significantly increased the synaptic efficacy as measured by EPSP amplitude 24 h after 5-HT treatment only in Ap-eIF3e overexpressing synapses (% change in EPSP amplitude, 150.2 ± 9.9, n = 17 and 105.7 ± 8.9, n = 14 for Ap-eIF3e overexpressing and control synapses, respectively; **, P < 0.01) (Fig. 3B). These data suggests that Ap-eIF3e is a positive regulator, which consolidates STF into LTF.
Fig. 3.
Effect of Ap-eIF3e overexpression and knock-down on 5-HT induced LTF. (A) Bar graph representing the effect of overexpression of Ap-eIF3e on basal synaptic transmission. Overexpression of Ap-eIF3e by injection of pNEXδ-Ap-eIF3e into the sensory cell did not produce any significant changes in synaptic strength. The height of each bar corresponds to the mean percentage of change ± SEM in EPSP amplitude tested 24 h after microinjection. (B) Bar graph representing the effect of overexpression of Ap-eIF3e on LTF. Overexpression of Ap-eIF3e in the sensory cell produced LTF when it was combined with the application of 10 μM 5-HT for 5 min (one pulse, 1×) that can induce only STF. The height of each bar corresponds to the mean percentage of change ± SEM in EPSP amplitude tested 24 h after 5-HT (10 μM) treatment. **, P < 0.01 (C) Effect of Ap-eIF3e dsRNA on basal synaptic transmission. EPSP amplitudes were measured 24 h after Ap-eIF3e or luciferase dsRNA injections. (D) Effect of Ap-eIF3e dsRNA on 5 × 5-HT-induced LTF. EPSP amplitudes were measured 24 h after 5× 5-HT treatment *, P < 0.05.
To investigate whether Ap-eIF3e is a necessary factor for LTF, expression of Ap-eIF3e was blocked by microinjecting dsRNA against Ap-eIF3e in a sensory neuron and then, five pulses of 5-HT were applied. Knock-down of Ap-eIF3e did not produce any significant change in the basal synaptic transmission one day after microinjection (% change in EPSP amplitude, 103.6 ± 3.9, n = 15 and 97.7 ± 5.0, n = 13 for Ap-eIF3e dsRNA and control luciferase dsRNA, respectively; P = 0.351, Fig. 3C). However, synapses injected with Ap-eIF3e dsRNA showed decreased facilitation 24 h after five pulses of 5-HT (% change in EPSP amplitude, 134.8 ± 10.7, n = 15; Fig. 3D). This facilitation was significantly different from the increase in EPSP amplitude measured in control dsRNA-injected synapses treated with five pulses of 5-HT (% change in EPSP amplitude, 171.2 ± 11.6, n = 13; *, P < 0.05, student's t test; Fig. 3D). These data suggest that Ap-eIF3e plays a critical role in the consolidation of LTF.
Discussion
We have generated and analyzed 11,493 A. kurodai ESTs. Although over thirty-five species of the genus Aplysia have been reported throughout the world, only one species A. californica has been studied extensively because it is commonly available along the US coasts and has been successfully bred in the laboratory (21, 22). A. kurodai is another commonly found species in the Asian Pacific area, along the Korean and Japanese coasts. Interestingly, although A. kurodai and A. californica are classified into two different subgenera Varria and Neoaplysia, respectively, the nervous system of A. kurodai appears almost identical to A. californica both anatomically and physiologically (6, 22). Furthermore, the molecular mechanisms underlying synaptic plasticity are well conserved in both species. For example, the neurotransmitter 5-HT can induce both short- and long-term synaptic facilitation in both species, depending on the number of treatments. In addition, common sets of signaling molecules and pathways play essentially identical roles in synaptic facilitation in both species (23–26).
Despite those similarities between two species and of the relatively larger size of EST database of A. californica, the current analysis shows that >30% of A. kurodai ESTs do not have homologous sequences in A. californica (Fig. 1). However, this might be an overestimate because of the limited genomic information from mollusks. A. kurodai transcripts, which had no A. californica matches or no BLAST matches, can be divided into three groups: (i) the genuine A. kurodai specific genes, (ii) noncoding RNAs, and (iii) intron related sequences. The data showing that 80% of A. californica nonmatching transcripts did not find matches in protein databases across all phyla (Fig. 1B) strongly suggests that most of the nonmatching sequences are from noncoding regions, which are less conserved or not sequenced in A. californica yet. In addition, of the remaining 20% BLAST-matching transcripts, only 3% had matches to known protein sequences from Mollusca, while 59% were homologous to vertebrates (Fig. 1C), which might be because of the incompleteness of molluscan databases. Taken together, it is less likely that the 33% of A. californica nonmatching transcripts are genuine A. kurodai-specific genes. We expect that an ongoing A. californica whole genome project will improve the quality of the ortholog-mapping procedure in the near future. Indeed, addition of the A. kurodai EST resources described here should facilitate the assembly and annotation of any forthcoming genomic sequences.
Because 5-HT is critically involved in both memory and synaptic facilitation in Aplysia, there has been a substantial amount of effort to identify differentially transcribed genes in response to this key neurotransmitter (11, 12, 27–29). In the present study, we used a microarray technique as a primary screening to identify 5-HT-regulated genes in Aplysia pleural ganglion. We monitored the gene expression profile 2 h after the onset of 5-HT treatment, which is a critical time period for the synthesis of macromolecules required for LTF (10). To validate the results of the primary screening and exclude false positives for the further studies, we performed qRT-PCR analysis. Because we used the low stringent experimental conditions in the primary screening (e.g., small number of arrays, no dye-swapping) to allow more chances to detect as many novel 5-HT responsive genes as possible, relatively large number of genes from the primary screening turned out to be false positives (Table 2). Nevertheless, we could identify a set of interesting 5-HT-regulated genes, which might be involved in synaptic facilitation.
The expression level of matrilin was increased in 5-HT treated pleural ganglia to more than fivefold than that of the control (Table 2 and Fig. 2). Matrilin belongs to a family of fibril-forming extracellular matrix proteins with four paralogues in the human genome (30). It participates in the formation of fibrillar or filamentous structures and is often associated with collagens. Moreover, it mediates interactions between collagen-containing fibrils and other matrix constituents (31). Although the role of matrilin in the nervous system has never been demonstrated, recent evidence suggests that extracellular matrix molecules, such as laminin, reelin and tenascins, are involved in synaptic plasticity by interacting with ion channels or neurotransmitter receptors (32). Another gene antistasin that also indirectly regulates cytoskeleton structure was found to be up-regulated (33), suggesting that up-regulation of matrilin and antistasin may play a role in synaptic plasticity, possibly by regulating cytoskeleton structures or other membrane proteins. In addition, BAT1 homolog was down-regulated by 5-HT treatment and confirmed by qRT-PCR (Fig. 2). BAT1 is known as an essential RNA splicing factor and also plays an important role in exporting mRNA from nucleus (34, 35). Aplysia BAT1 homolog may regulate mRNA transport and/or splicing of some synaptic plasticity-related genes. The role of this down-regulated gene in LTF remains to be investigated.
To examine the possible role of differentially expressed genes in synaptic facilitation, we performed a functional analysis on one of the up-regulated genes, Ap-eIF3e. Previously, we have shown that the overexpression of an immediate early gene ApC/EBP in presynaptic sensory neuron enables a single pulse of 5-HT to induce LTF that is normally achieved by repeated five pulses of 5-HT in sensory to motor coculture (25). Similarly, the overexpression of Ap-eIF3e also converted STF to LTF. Moreover, when Ap-eIF3e expression was blocked by dsRNA, LTF induction by repeated pulses of 5-HT was impaired, suggesting that Ap-eIF3e is critically involved in consolidation of LTF. eIF3e/Int6 was initially characterized as a common integration site for the mouse mammary tumor virus (MMTV) in mouse mammary tumors (36). MMTV insertion into Int6 causes the production of a truncated mutant protein, which has the transforming activity, suggesting that normal Int6 functions as a tumor suppressor gene (37). Although it was identified as a subunit homolog of eIF3, which binds to 40S ribosomal subunit (38), it is not essential for translation and thought to play a regulatory role (39). Several lines of evidence suggest that eIF3e/Int6 is also involved in protein degradation by interacting with proteasome complexes (40, 41). Recently, eIF3e/Int6 was shown to be involved in activity-dependent internalization of voltage-gated calcium channel in cortical neuron (42). So far, the molecular function of Ap-eIF3e is unclear. Does Ap-eIF3e facilitate the translation of LTF-related proteins? Is it involved in the degradation of negative regulatory proteins during consolidation of LTF? These issues will be addressed in future experiments.
In summary, by constructing, annotating and comparatively analyzing A. kurodai ESTs with A. californica ESTs, our data provides a complementary resource to the Aplysia genome annotation. Moreover, we identified Ap-eIF3e as a positive regulator in 5-HT-induced LTF. Further functional studies of other differentially expressed genes found in the present study will provide insights to improve our understanding of the molecular mechanisms underlying learning and memory.
Materials and Methods
cDNA Library Construction.
The total RNA was isolated from A. kurodai central nervous system (CNS) with TRIzol (Invitrogen), followed by the purification of poly (A)+ RNA (Oligotex mRNA midi kit; Qiagen). Five libraries were used in this study. A random-primed library was constructed by using a random primer (9mer) with a Takara cDNA synthesis kit (Takara) following the manufacturer's manual. cDNA inserts were flanked by a linker containing a BstXI enzyme site and cloned into BstXI-digested pYesTrp2 vector (Invitrogen). Full-length-enriched and 5′-end-enriched libraries were constructed as previously described (13, 15). Briefly, oligo-capping was performed after the purification of poly (A)+ RNA. After removing unligated 5′-oligo, cDNA was synthesized by using dT adapter-primer (full-length-enriched) or random adapter-primer (5′-end-enriched) and amplified by PCR. PCR products were digested with SfiI and cloned into DraIII-digested pME18S-FL vector. The 5-HT-treated CNS library was constructed from mRNA, which was extracted from CNS of in vivo 5-HT-treated animals (250 μM for 2 h). cDNA was synthesized by using oligo(dT) as a primer and adapted with EcoRI linker. Then, the cDNA was directionally cloned into Uni-ZAP XR vector (Stratagene). The buccal muscle library was constructed with mRNA from Aplysia buccal muscle by following the same procedure as the 5-HT-treated library using Uni-ZAP XR cloning system (Stratagene).
EST Sequencing, Assembly, and Annotation.
Plasmid DNA was extracted from the libraries and sequenced by using a 3730xl DNA analyzer (Applied Biosystems). The sequencing primers used were 5′-TGGATGTTGCCTTTACTTCT-3′ (full-length-enriched and 5′-end-enriched libraries) and 5′-GCGTGAATGTAAGCGTGAC-3′ (random-primed library), respectively. 5-HT-treated and buccal muscle libraries were sequenced with T3 primer. Base-calling and quality assessment were performed with phred (43, 44). Vector sequences were trimmed by using the sequence comparison program cross_match (45). EST clustering was performed by BLAST (18), and then clustered through a single-linkage method (46). Each clustered EST file was assembled by using the contig assembly program CAP3 (16). Gene ontology format files and gene association files of the Compugen gene ontology (17) were obtained from the GO database website (www.geneontology.org). We extracted information about the relationships of the GO terms through the hierarchical structure of gene ontology. To classify putative transcripts of A. kurodai into GO categories, these sequences were searched against the GO protein sets of Compugen's GenBank GO annotation by using BLAST.
Microarray and qRT-PCR.
The construction and hybridization of the cDNA microarray were performed as described previously (47). Please see SI Materials and Methods for the details.
Cloning and Microinjection of Ap-eIF3e cDNA.
cDNA showing a high homology to eIF3e (5HTCNS122105-T3-C10–628) was rescued from an A. kurodai 5-HT-treated cDNA library. The 5′-end of the cDNA including the start codon was obtained by performing 5′-RACE using the SMART-RACE cDNA amplification kit (Clontech). The full-length Ap-eIF3e CDS was amplified by PCR using A. Kurodai pleural gangla cDNA as templates. The amplified CDS was subcloned into a HindIII-XbaI site of expression vector pNEXδ (4). To generate dsRNA, a XhoI-SacI fragment of 5HTCNS122105-T3-C10–628 (≈800 bp) was subcloned into pLitmus28i (New England Biolabs). Sense and antisense RNAs were synthesized by in vitro transcription of linearized template DNAs using T7 RNA polymerase (Ambion) and annealed. Microinjection was primarily conducted as described previously (4). The DNA (pNEXδ-Ap-eIF3e, 500 ng/μL) or dsRNA (500 ng/μL) was mixed with a reporter construct pNEXδ-EGFP (500 ng/μL) in the injection solution (10 mM Tris-Cl; 100 mM NaCl; and 0.1%fast green; pH 7.3) immediately before the injection.
Sensory-to-Motor Coculture and Electrophysiology.
Sensory-to-motor neuron coculture of A. kurodai and electrophysiological recording of 5-HT-induced long-term facilitation were performed as described previously by Lee and colleagues (25). The percent change in EPSP amplitude of basal synaptic transmission was measured by comparing EPSP amplitude at 24 h after DNA or dsRNA microinjection with initial EPSP amplitude. To induce long-term facilitation in sensory-to-motor synapse, five pulses of 5-HT (10 μM) were treated for 5 min at 15-min intervals. The resultant percent change in EPSP amplitude was calculated by comparing EPSP amplitude at 24 h after 5-HT treatment to that before 5-HT treatment.
Supplementary Material
Acknowledgments.
This work was supported by the National Creative Research Initiative Program of the Korean Ministry of Science and Technology and the Marine and Extreme Genome Research Center Program, Ministry of Marine Affairs and Fisheries, Republic of Korea. Korean Bio Information Center was supported by the Korean Ministry of Science and Technology under grant number M10407010001-04N0701-00110 and by the Ministry of Information and Communication, Korea, under the Korea Agency Digital Opportunity and Promotion support program (06-121). Y.-S.L., S.-L.C., and D.-J.J. are supported by BK21 fellowships. L.M. is supported by National Institutes of Health, National Science Foundation, McKnight Brain Research Foundation, and University of Florida opportunity funds. E.K. is supported by the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808893105/DCSupplemental.
References
- 1.Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carew TJ, Sahley CL. Invertebrate learning and memory: From behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- 3.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 4.Kaang BK. Parameters influencing ectopic gene expression in Aplysia neurons. Neurosci Lett. 1996;221:29–32. doi: 10.1016/s0304-3940(96)13279-1. [DOI] [PubMed] [Google Scholar]
- 5.Kandel ER. Cellular basis of behavior. New York: W.H. Freeman and company; 1976. [Google Scholar]
- 6.Lim CS, Chung DY, Kaang BK. Partial anatomical and physiological characterization and dissociated cell culture of the nervous system of the marine mollusc Aplysia kurodai. Mol Cells. 1997;7:399–407. [PubMed] [Google Scholar]
- 7.Moccia R, et al. An unbiased cDNA library prepared from isolated Aplysia sensory neuron processes is enriched for cytoskeletal and translational mRNAs. J Neurosci. 2003;23:9409–9417. doi: 10.1523/JNEUROSCI.23-28-09409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen B, Kohn AB, Nahir B, McFadden CS, Moroz LL. Complete DNA sequence of the mitochondrial genome of the sea-slug, Aplysia californica: Conservation of the gene order in Euthyneura. Mol Phylogenet Evol. 2006;38:459–469. doi: 10.1016/j.ympev.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Moroz LL, et al. Neuronal transcriptome of aplysia: Neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montarolo PG, et al. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 11.Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 12.Hegde AN, et al. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 1997;89:115–126. doi: 10.1016/s0092-8674(00)80188-9. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Sugano S. Construction of a full-length enriched and a 5′-end enriched cDNA library using the oligo-capping method. Methods Mol Biol. 2003;221:73–91. doi: 10.1385/1-59259-359-3:73. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S. Construction and characterization of a full length-enriched and a 5′-end-enriched cDNA library. Gene. 1997;200:149–156. doi: 10.1016/s0378-1119(97)00411-3. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Sugano S. Construction of full-length-enriched cDNA libraries. The oligo-capping method. Methods Mol Biol. 2001;175:143–153. doi: 10.1385/1-59259-235-X:143. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie H, et al. Large-scale protein annotation through gene ontology. Genome Res. 2002;12:785–794. doi: 10.1101/gr.86902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burks EA, Bezerra PP, Le H, Gallie DR, Browning KS. Plant initiation factor 3 subunit composition resembles mammalian initiation factor 3 and has a novel subunit. J Biol Chem. 2001;276:2122–2131. doi: 10.1074/jbc.M007236200. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann K, Bucher P. The PCI domain: A common theme in three multiprotein complexes. Trends Biochem Sci. 1998;23:204–205. doi: 10.1016/s0968-0004(98)01217-1. [DOI] [PubMed] [Google Scholar]
- 21.Kriegstein AR, Castellucci V, Kandel ER. Metamorphosis of Aplysia californica in laboratory culture. Proc Natl Acad Sci USA. 1974;71:3654–3658. doi: 10.1073/pnas.71.9.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandel ER. Behavioral Biology of Aplysia: A Contribution to the Comparative Study of Opisthobranch Molluscs. San Francisco: W. H. Freeman; 1979. [Google Scholar]
- 23.Han JH, Lim CS, Lee YS, Kandel ER, Kaang BK. Role of Aplysia cell adhesion molecules during 5-HT-induced long-term functional and structural changes. Learn Mem. 2004;11:421–435. doi: 10.1101/lm.61104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang DJ, et al. Activation of a heterologously expressed octopamine receptor coupled only to adenylyl cyclase produces all the features of presynaptic facilitation in aplysia sensory neurons. Proc Natl Acad Sci USA. 2000;97:1829–1834. doi: 10.1073/pnas.97.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JA, et al. Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses. Learn Mem. 2001;8:220–226. doi: 10.1101/lm.40201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JA, Kim H, Lee YS, Kaang BK. Overexpression and RNA interference of Ap-cyclic AMP-response element binding protein-2, a repressor of long-term facilitation, in Aplysia kurodai sensory-to-motor synapses. Neurosci Lett. 2003;337:9–12. doi: 10.1016/s0304-3940(02)01285-5. [DOI] [PubMed] [Google Scholar]
- 27.Barzilai A, Kennedy TE, Sweatt JD, Kandel ER. 5-HT modulates protein synthesis and the expression of specific proteins during long-term facilitation in Aplysia sensory neurons. Neuron. 1989;2:1577–1586. doi: 10.1016/0896-6273(89)90046-9. [DOI] [PubMed] [Google Scholar]
- 28.Noel F, Nunez-Regueiro M, Cook R, Byrne JH, Eskin A. Long-term changes in synthesis of intermediate filament protein, actin and other proteins in pleural sensory neurons of Aplysia produced by an in vitro analogue of sensitization training. Brain Res Mol Brain Res. 1993;19:203–210. doi: 10.1016/0169-328x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- 29.Liu QR, et al. A developmental gene (Tolloid/BMP-1) is regulated in Aplysia neurons by treatments that induce long-term sensitization. J Neurosci. 1997;17:755–764. doi: 10.1523/JNEUROSCI.17-02-00755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deak F, Wagener R, Kiss I, Paulsson M. The matrilins: A novel family of oligomeric extracellular matrix proteins. Matrix Biol. 1999;18:55–64. doi: 10.1016/s0945-053x(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 31.Wagener R, et al. The matrilins–adaptor proteins in the extracellular matrix. FEBS Lett. 2005;579:3323–3329. doi: 10.1016/j.febslet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- 33.Leadley RJ, Jr, Chi L, Porcari AR. Non-hemostatic activity of coagulation factor Xa: Potential implications for various diseases. Curr Opin Pharmacol. 2001;1:169–175. doi: 10.1016/s1471-4892(01)00033-9. [DOI] [PubMed] [Google Scholar]
- 34.Fleckner J, Zhang M, Valcarcel J, Green MR. U2AF65 recruits a novel human DEAD box protein required for the U2 snRNP-branchpoint interaction. Genes Dev. 1997;11:1864–1872. doi: 10.1101/gad.11.14.1864. [DOI] [PubMed] [Google Scholar]
- 35.Herold A, Teixeira L, Izaurralde E. Genome-wide analysis of nuclear mRNA export pathways in Drosophila. EMBO J. 2003;22:2472–2483. doi: 10.1093/emboj/cdg233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchetti A, et al. Int-6, a highly conserved, widely expressed gene, is mutated by mouse mammary tumor virus in mammary preneoplasia. J Virol. 1995;69:1932–1938. doi: 10.1128/jvi.69.3.1932-1938.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen SB, Kordon E, Callahan R, Smith GH. Evidence for the transforming activity of a truncated Int6 gene in vitro. Oncogene. 2001;20:5291–5301. doi: 10.1038/sj.onc.1204624. [DOI] [PubMed] [Google Scholar]
- 38.Asano K, Merrick WC, Hershey JW. The translation initiation factor eIF3–p48 subunit is encoded by int-6, a site of frequent integration by the mouse mammary tumor virus genome. J Biol Chem. 1997;272:23477–23480. doi: 10.1074/jbc.272.38.23477. [DOI] [PubMed] [Google Scholar]
- 39.Bandyopadhyay A, Matsumoto T, Maitra U. Fission yeast Int6 is not essential for global translation initiation, but deletion of int6(+) causes hypersensitivity to caffeine and affects spore formation. Mol Biol Cell. 2000;11:4005–4018. doi: 10.1091/mbc.11.11.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yen HC, Gordon C, Chang EC. Schizosaccharomyces pombe Int6 and Ras homologs regulate cell division and mitotic fidelity via the proteasome. Cell. 2003;112:207–217. doi: 10.1016/s0092-8674(03)00043-6. [DOI] [PubMed] [Google Scholar]
- 41.von Arnim AG, Chamovitz DA. Protein homeostasis: A degrading role for Int6/eIF3e. Curr Biol. 2003;13:R323–325. doi: 10.1016/s0960-9822(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 42.Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE. The tumor suppressor eIF3e mediates calcium-dependent internalization of the l-type calcium channel CaV1.2. Neuron. 2007;55:615–632. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- 44.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 45.Gordon D, Abajian C, Green P. Consed: A graphical tool for sequence finishing. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- 46.Pertea G, et al. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics. 2003;19:651–652. doi: 10.1093/bioinformatics/btg034. [DOI] [PubMed] [Google Scholar]
- 47.Hegde P, et al. A concise guide to cDNA microarray analysis. Biotechniques. 2000;29:548–556. doi: 10.2144/00293bi01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.