Abstract
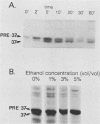
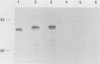
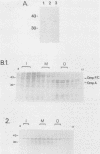
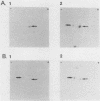
We previously reported the complete primary structure of the 37-kilodalton endoflagellar sheath protein (FlaA) of Treponema pallidum. However, we were unable to determine the nucleotide sequence of flaA upstream of amino acid 10. The desired nucleotide sequence was obtained by use of a strategy based upon the polymerase chain reaction and was found to contain a consensus Escherichia coli promoter, a ribosomal binding site, and a 20-amino-acid signal peptide. Expression of FlaA in E. coli was achieved by cloning polymerase chain reaction-derived constructs lacking the native T. pallidum promoter into a temperature-inducible T7 expression system. Pulse-chase and ethanol inhibition analyses of protein processing in E. coli cells and minicells, respectively, indicated that processing of the FlaA precursor was incomplete. Native and recombinant FlaA were identical as assessed by antibody reactivity and sodium dodecyl sulfate- and two-dimensional polyacrylamide gel electrophoretic mobilities. Soluble FlaA was not detected in either the cytoplasmic or the periplasmic fractions of E. coli transformants. Fractionation of E. coli cell envelopes unexpectedly revealed that FlaA precursor and FlaA were associated with both the cytoplasmic and outer membranes. This is the first report of expression in E. coli of a T. pallidum protein which could not be cloned or expressed with its native promoter. Our data also indicate that information obtained in E. coli regarding the subcellular location of cloned treponemal proteins must be cautiously extrapolated to T. pallidum.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anba J., Lazdunski C., Pages J. M. Lack of effect of leader peptidase overproduction on the processing in vivo of exported proteins in Escherichia coli. J Gen Microbiol. 1986 Mar;132(3):689–696. doi: 10.1099/00221287-132-3-689. [DOI] [PubMed] [Google Scholar]
- Bailey M. J., Thomas C. M., Cockayne A., Strugnell R. A., Penn C. W. Cloning and expression of Treponema pallidum antigens in Escherichia coli. J Gen Microbiol. 1989 Sep;135(9):2365–2378. doi: 10.1099/00221287-135-9-2365. [DOI] [PubMed] [Google Scholar]
- Baker-Zander S. A., Fohn M. J., Lukehart S. A. Development of cellular immunity to individual soluble antigens of Treponema pallidum during experimental syphilis. J Immunol. 1988 Dec 15;141(12):4363–4369. [PubMed] [Google Scholar]
- Baker-Zander S. A., Hook E. W., 3rd, Bonin P., Handsfield H. H., Lukehart S. A. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J Infect Dis. 1985 Feb;151(2):264–272. doi: 10.1093/infdis/151.2.264. [DOI] [PubMed] [Google Scholar]
- Beckwith J., Silhavy T. J. Genetic analysis of protein export in Escherichia coli. Methods Enzymol. 1983;97:3–11. doi: 10.1016/0076-6879(83)97114-8. [DOI] [PubMed] [Google Scholar]
- Bernadac A., Bolla J. M., Lazdunski C., Inouye M., Pages J. M. Precise localization of an overproduced periplasmic protein in Escherichia coli: use of double immuno-gold labelling. Biol Cell. 1987;61(3):141–147. doi: 10.1111/j.1768-322x.1987.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Blanco D. R., Champion C. I., Miller J. N., Lovett M. A. Antigenic and structural characterization of Treponema pallidum (Nichols strain) endoflagella. Infect Immun. 1988 Jan;56(1):168–175. doi: 10.1128/iai.56.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Sparling P. F. Molecular cloning and characterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9084–9088. doi: 10.1073/pnas.84.24.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., Radolf J. D., Hsu P. L., Sell S., Norgard M. V. Genetic and physicochemical characterization of the recombinant DNA-derived 47-kilodalton surface immunogen of Treponema pallidum subsp. pallidum. Infect Immun. 1988 Jan;56(1):71–78. doi: 10.1128/iai.56.1.71-78.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne A., Bailey M. J., Penn C. W. Analysis of sheath and core structures of the axial filament of Treponema pallidum. J Gen Microbiol. 1987 Jun;133(6):1397–1407. doi: 10.1099/00221287-133-6-1397. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Ray P. H., Leong J., Benedict C. D., Stamm L. V., Bassford P. J., Jr Identification and purification of a recombinant Treponema pallidum basic membrane protein antigen expressed in Escherichia coli. Infect Immun. 1987 May;55(5):1106–1115. doi: 10.1128/iai.55.5.1106-1115.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Radolf J. D., Walfield A. M., Cunningham T. M., Miller J. N., Lovett M. A. Native surface association of a recombinant 38-kilodalton Treponema pallidum antigen isolated from the Escherichia coli outer membrane. Infect Immun. 1986 May;52(2):586–593. doi: 10.1128/iai.52.2.586-593.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M. E., Blake M. S., Koomey M. Porin protein of Neisseria gonorrhoeae: cloning and gene structure. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8135–8139. doi: 10.1073/pnas.84.22.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatelli J. C., Gingeras T. R., Richman D. D. Nucleic acid amplification in vitro: detection of sequences with low copy numbers and application to diagnosis of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 1989 Apr;2(2):217–226. doi: 10.1128/cmr.2.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Verduzco L. M., Kupersztoch Y. M. Fusion of Escherichia coli heat-stable enterotoxin and heat-labile enterotoxin B subunit. J Bacteriol. 1987 Nov;169(11):5201–5208. doi: 10.1128/jb.169.11.5201-5208.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Bishop N. H., Miller J. N., Lovett M. A. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J Immunol. 1983 Oct;131(4):1973–1977. [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Pedersen P. E., Schouls L. M., Severin E., van Embden J. D. Genetic characterization and partial sequence determination of a Treponema pallidum operon expressing two immunogenic membrane proteins in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1227–1237. doi: 10.1128/jb.162.3.1227-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Gonzales F. R., Chamberlain N. R., Norgard M. V., Miller E. E., Cope L. D., Pelzel S. E., Gaddy B., Clausell A. Cloning of the gene encoding the major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1988 Oct;56(10):2709–2716. doi: 10.1128/iai.56.10.2709-2716.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougen K. H. Further observations on the ultrastructure of Treponema pallidum nichols. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(2):297–304. doi: 10.1111/j.1699-0463.1972.tb00163.x. [DOI] [PubMed] [Google Scholar]
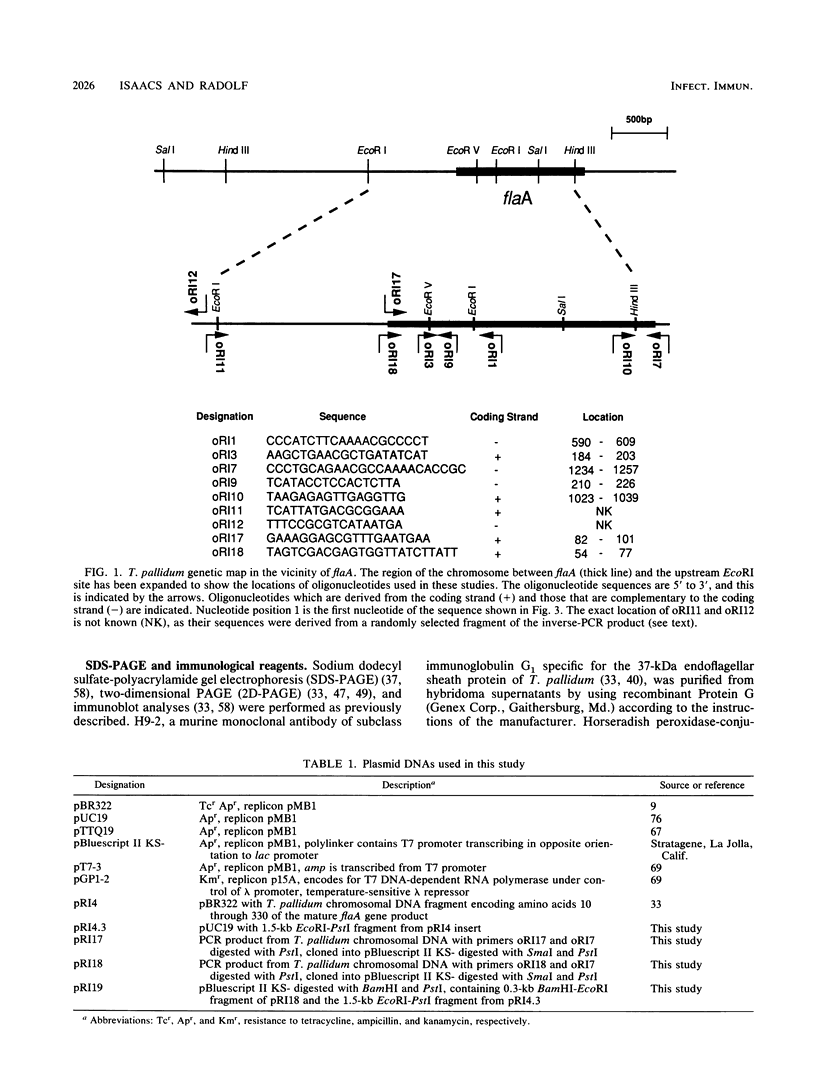
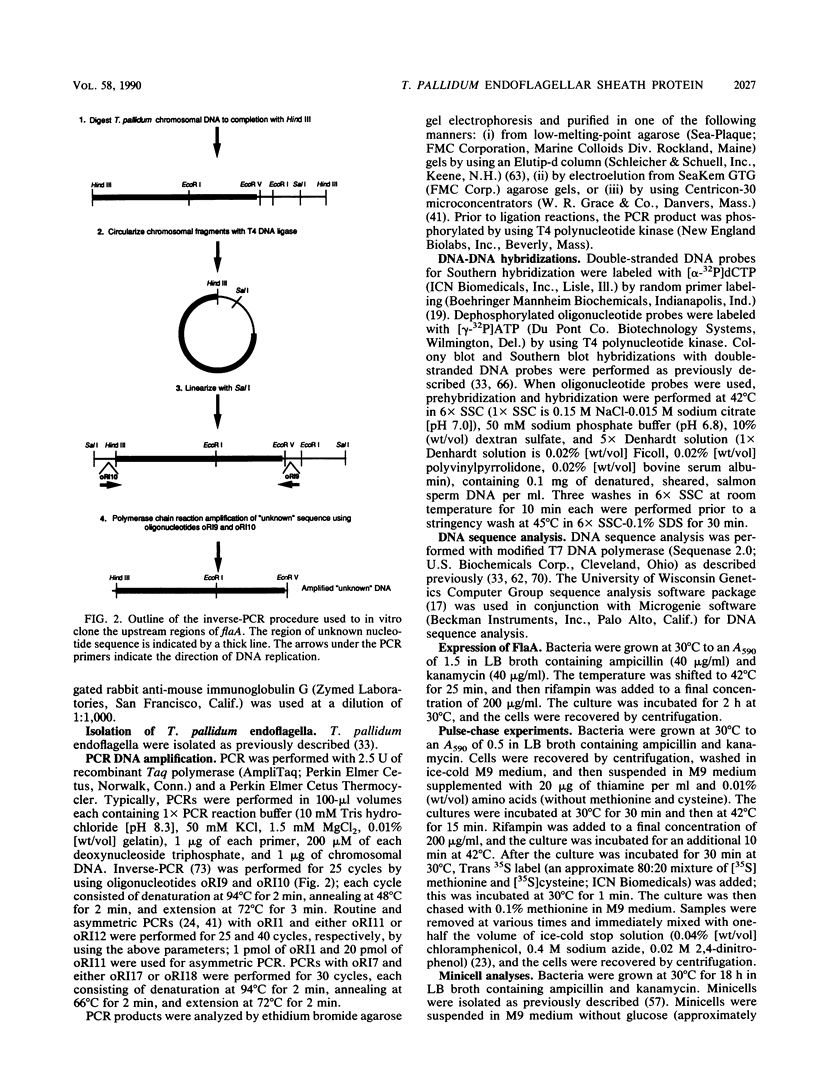
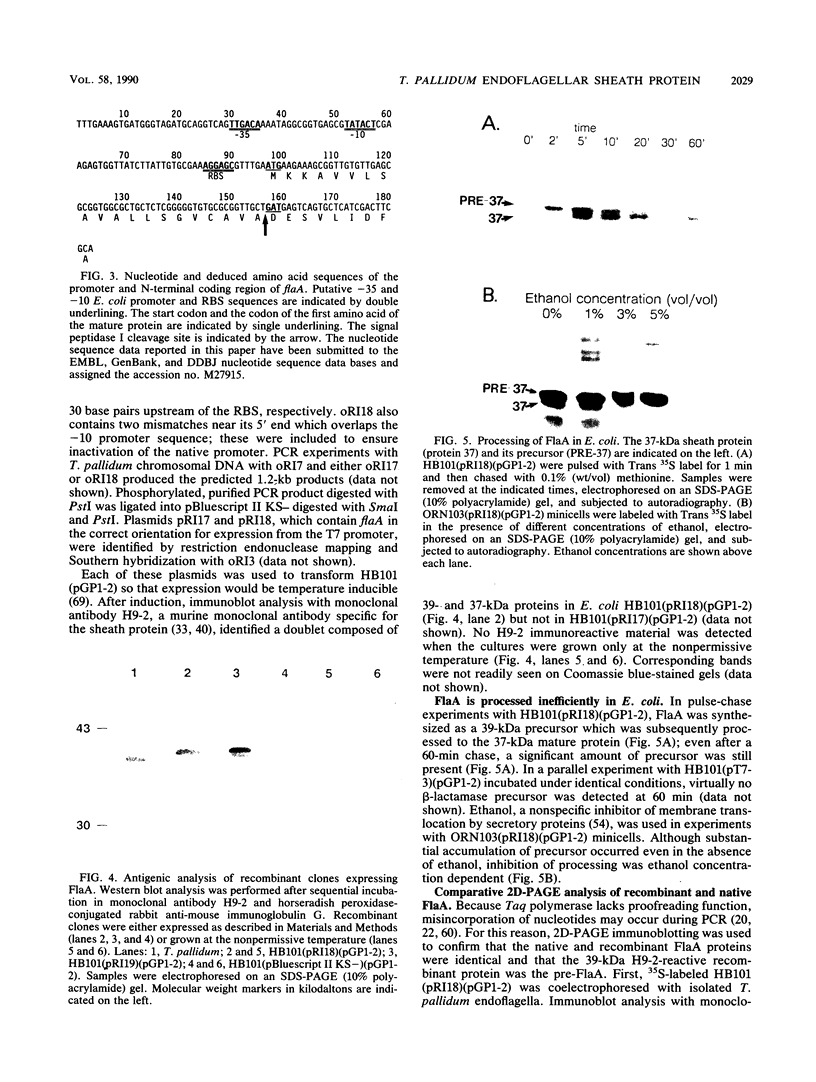
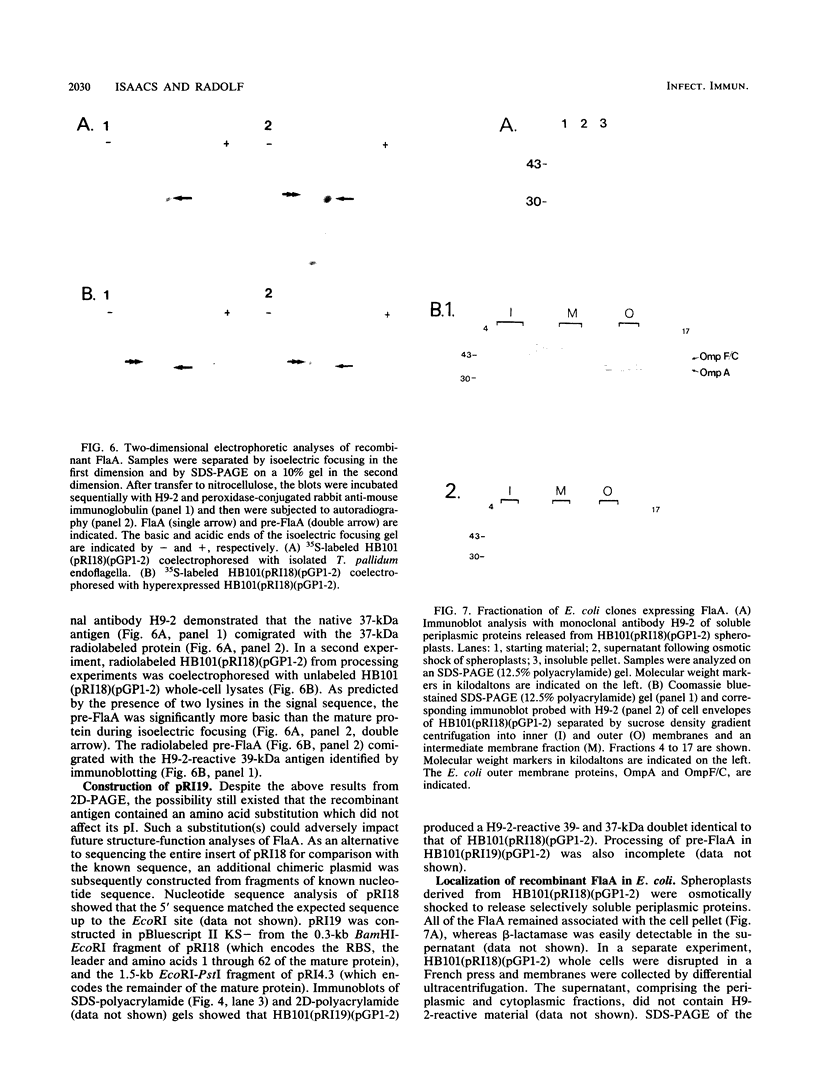
- Isaacs R. D., Hanke J. H., Guzman-Verduzco L. M., Newport G., Agabian N., Norgard M. V., Lukehart S. A., Radolf J. D. Molecular cloning and DNA sequence analysis of the 37-kilodalton endoflagellar sheath protein gene of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3403–3411. doi: 10.1128/iai.57.11.3403-3411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Kuriki Y. Response to temperature shifts of expression of the amp gene on pBR322 in Escherichia coli K-12. J Bacteriol. 1987 May;169(5):2294–2297. doi: 10.1128/jb.169.5.2294-2297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima G., Kawagishi I., Homma M., Asaka J., Kondo E., Macnab R. M. Export of an N-terminal fragment of Escherichia coli flagellin by a flagellum-specific pathway. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4953–4957. doi: 10.1073/pnas.86.13.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Limberger R. J., Charon N. W. Treponema phagedenis has at least two proteins residing together on its periplasmic flagella. J Bacteriol. 1986 Apr;166(1):105–112. doi: 10.1128/jb.166.1.105-112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of the humoral immune response of the rabbit to antigens of Treponema pallidum after experimental infection and therapy. Sex Transm Dis. 1986 Jan-Mar;13(1):9–15. doi: 10.1097/00007435-198601000-00003. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Tam M. R., Hom J., Baker-Zander S. A., Holmes K. K., Nowinski R. C. Characterization of monoclonal antibodies to Treponema pallidum. J Immunol. 1985 Jan;134(1):585–592. [PubMed] [Google Scholar]
- Mihovilovic M., Lee J. E. An efficient method for sequencing PCR amplified DNA. Biotechniques. 1989 Jan;7(1):14–16. [PubMed] [Google Scholar]
- Morita T., Amemura M., Makino K., Shinagawa H., Magota K., Otsuji N., Nakata A. Hyperproduction of phosphate-binding protein, phoS, and pre-phoS proteins in Escherichia coli carrying a cloned phoS gene. Eur J Biochem. 1983 Feb 15;130(3):427–435. doi: 10.1111/j.1432-1033.1983.tb07169.x. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Tolan R. W., Jr Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989 Jan;57(1):88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Cloning and expression of Treponema pallidum (Nichols) antigen genes in Escherichia coli. Infect Immun. 1983 Nov;42(2):435–445. doi: 10.1128/iai.42.2.435-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Charon N. W., Cook R. G., Fuentes M. D., Limberger R. J. Antigenic relatedness and N-terminal sequence homology define two classes of periplasmic flagellar proteins of Treponema pallidum subsp. pallidum and Treponema phagedenis. J Bacteriol. 1988 Sep;170(9):4072–4082. doi: 10.1128/jb.170.9.4072-4082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Nucleotide sequence of pilA, the gene encoding the structural component of type 1 pili in Escherichia coli. J Bacteriol. 1985 Apr;162(1):454–457. doi: 10.1128/jb.162.1.454-457.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pages J. M., Anba J., Bernadac A., Shinagawa H., Nakata A., Lazdunski C. Normal precursors of periplasmic proteins accumulated in the cytoplasm are not exported post-translationally in Escherichia coli. Eur J Biochem. 1984 Sep 17;143(3):499–505. doi: 10.1111/j.1432-1033.1984.tb08398.x. [DOI] [PubMed] [Google Scholar]
- Pallesen L., Hindersson P. Cloning and sequencing of a Treponema pallidum gene encoding a 31.3-kilodalton endoflagellar subunit (FlaB2). Infect Immun. 1989 Jul;57(7):2166–2172. doi: 10.1128/iai.57.7.2166-2172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Hirst T. R., Hardy S. J., Holmgren J., Randall L. Synthesis of a precursor to the B subunit of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1981 Apr;146(1):325–330. doi: 10.1128/jb.146.1.325-330.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Involvement of periplasmic fibrils in motility of spirochetes. J Bacteriol. 1980 Jan;141(1):359–364. doi: 10.1128/jb.141.1.359-364.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Bailey M. J., Cockayne A. The axial filament antigen of Treponema pallidum. Immunology. 1985 Apr;54(4):635–641. [PMC free article] [PubMed] [Google Scholar]
- Purcell B. K., Chamberlain N. R., Goldberg M. S., Andrews L. P., Robinson E. J., Norgard M. V., Radolf J. D. Molecular cloning and characterization of the 15-kilodalton major immunogen of Treponema pallidum. Infect Immun. 1989 Dec;57(12):3708–3714. doi: 10.1128/iai.57.12.3708-3714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Schulz W. W. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J. J., Cohen B. N. Quantitative isolation of DNA restriction fragments from low-melting agarose by Elutip-d affinity chromatography. Anal Biochem. 1983 Sep;133(2):462–464. doi: 10.1016/0003-2697(83)90109-4. [DOI] [PubMed] [Google Scholar]
- Schouls L. M., Ijsselmuiden O. E., Weel J., van Embden J. D. Overproduction and purification of Treponema pallidum recombinant-DNA-derived proteins TmpA and TmpB and their potential use in serodiagnosis of syphilis. Infect Immun. 1989 Sep;57(9):2612–2623. doi: 10.1128/iai.57.9.2612-2623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark M. J. Multicopy expression vectors carrying the lac repressor gene for regulated high-level expression of genes in Escherichia coli. Gene. 1987;51(2-3):255–267. doi: 10.1016/0378-1119(87)90314-3. [DOI] [PubMed] [Google Scholar]
- Swancutt M. A., Riley B. S., Radolf J. D., Norgard M. V. Molecular characterization of the pathogen-specific, 34-kilodalton membrane immunogen of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3314–3323. doi: 10.1128/iai.57.11.3314-3323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Navab M., Haake D. A., Fogelman A. M., Miller J. N., Lovett M. A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. 1988 May;85(10):3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J. Fallacies of E. coli cell fractionations and consequences thereof for protein export models. Microb Pathog. 1986 Jun;1(3):225–228. doi: 10.1016/0882-4010(86)90046-x. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. M., Zampighi G. A., Blanco D. R., Miller J. N., Lovett M. A. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol. 1989 Sep;171(9):5005–5011. doi: 10.1128/jb.171.9.5005-5011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Embden J. D., van der Donk H. J., van Eijk R. V., van der Heide H. G., de Jong J. A., van Olderen M. F., Osterhaus A. B., Schouls L. M. Molecular cloning and expression of Treponema pallidum DNA in Escherichia coli K-12. Infect Immun. 1983 Oct;42(1):187–196. doi: 10.1128/iai.42.1.187-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]