Abstract
The role of antibody in protection against infection with Cryptococcus neoformans is undefined. In this paper we describe the development of opsonic activity in the cerebrospinal fluid (CSF) of rabbits in response to cryptococcal meningitis. The opsonin appeared to be immunoglobulin G (IgG); the activity was heat stable, copurified with the IgG fraction during protein A separation, and could be absorbed by encapsulated cryptococci. Immunosuppression with cyclosporine could be administered to prevent or allow in vivo deposition of IgG on the polysaccharide capsule of yeast in the CSF. Both early and late cyclosporine regimens resulted in prolonged, severe meningeal infections corresponding to the complete absence of in vitro opsonic activity in the CSF. While the production of opsonic antibody is part of the successful host response against C. neoformans in the central nervous system of rabbits, the presence of specific immunoglobulin by itself is insufficient for complete protection.
Full text
PDF


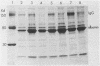
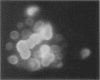
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–922. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985 Aug;76(2):508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974 Oct;14(1):12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Hague M., Drutz D. J. Passive immunization in murine cryptococcosis. Sabouraudia. 1981 Dec;19(4):237–244. doi: 10.1080/00362178185380411. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Taylor R. L. Host defense in cryptococcosis. I. An in vivo model for evaluating immune response. Int Arch Allergy Appl Immunol. 1978;57(2):101–113. [PubMed] [Google Scholar]
- Gupta S., Ellis M., Cesario T., Ruhling M., Vayuvegula B. Disseminated cryptococcal infection in a patient with hypogammaglobulinemia and normal T cell functions. Am J Med. 1987 Jan;82(1):129–131. doi: 10.1016/0002-9343(87)90388-3. [DOI] [PubMed] [Google Scholar]
- Keown P. A., Essery G. L., Stiller C. R., Sinclair N. R., Mullen R., Ulan R. A. Mechanisms of immunosuppression by cyclosporin. Transplant Proc. 1981 Mar;13(1 Pt 1):386–389. [PubMed] [Google Scholar]
- Kinnman J., Link H., Frydén A. Characterization of antibody activity in oligoclonal immunoglobulin G synthesized within the central nervous system in a patient with tuberculous meningitis. J Clin Microbiol. 1981 Jan;13(1):30–35. doi: 10.1128/jcm.13.1.30-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986 Apr;52(1):1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Mantia L., Salmaggi A., Tajoli L., Cerrato D., Lamperti E., Nespolo A., Bussone G. Cryptococcal meningoencephalitis: intrathecal immunological response. J Neurol. 1986 Nov;233(6):362–366. doi: 10.1007/BF00313923. [DOI] [PubMed] [Google Scholar]
- Leapman S. B., Filo R. S., Smith E. J., Smith P. G. Differential effects of cyclosporin-A on lymphocyte subpopulations. Transplant Proc. 1981 Mar;13(1 Pt 1):405–409. [PubMed] [Google Scholar]
- Louria D. B., Kaminski T. Passively-acquired immunity in experimental cryptococcosis. Sabouraudia. 1965 Jun;4(2):80–84. doi: 10.1080/00362176685190211. [DOI] [PubMed] [Google Scholar]
- Miller G. P., Kohl S. Antibody-dependent leukocyte killing of Cryptococcus neoformans. J Immunol. 1983 Sep;131(3):1455–1459. [PubMed] [Google Scholar]
- Müller F., Moskophidis M., Prange H. W. Demonstration of locally synthesized immunoglobulin M antibodies to Treponema pallidum in the central nervous system of patients with untreated neurosyphilis. J Neuroimmunol. 1984 Nov;7(1):43–54. doi: 10.1016/s0165-5728(84)80005-3. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Durack D. T. Effects of cyclosporine in experimental cryptococcal meningitis. Infect Immun. 1985 Oct;50(1):22–26. doi: 10.1128/iai.50.1.22-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Hobbs M. M., Granger D. L., Durack D. T. Cerebrospinal fluid macrophage response to experimental cryptococcal meningitis: relationship between in vivo and in vitro measurements of cytotoxicity. Infect Immun. 1988 Apr;56(4):849–854. doi: 10.1128/iai.56.4.849-854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Lang S. D., Durack D. T. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980 Oct;101(1):177–194. [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Lang S. D., Durack D. T. Influence of agglutinating antibody in experimental cryptococcal meningitis. Br J Exp Pathol. 1981 Dec;62(6):595–599. [PMC free article] [PubMed] [Google Scholar]
- Sabetta J. R., Andriole V. T. Cryptococcal infection of the central nervous system. Med Clin North Am. 1985 Mar;69(2):333–344. doi: 10.1016/s0025-7125(16)31046-x. [DOI] [PubMed] [Google Scholar]
- Vandvik B., Vartdal F., Norrby E. Herpes simplex virus encephalitis: intrathecal synthesis of oligoclonal virus-specific IgG, IgA and IgM antibodies. J Neurol. 1982;228(1):25–38. doi: 10.1007/BF00313407. [DOI] [PubMed] [Google Scholar]
- White D. J., Plumb A. M., Pawelec G., Brons G. Cyclosporin A: an immunosuppressive agent preferentially active against proliferating T cells. Transplantation. 1979 Jan;27(1):55–58. [PubMed] [Google Scholar]