Abstract
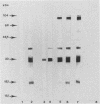
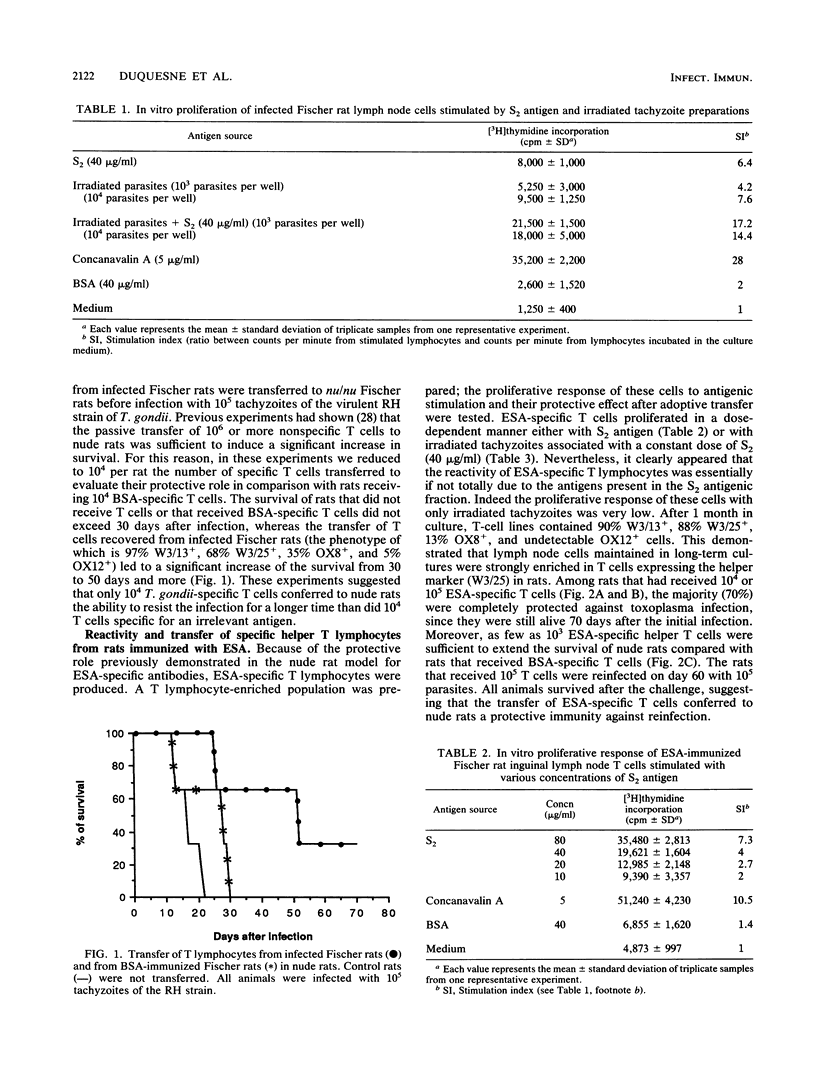
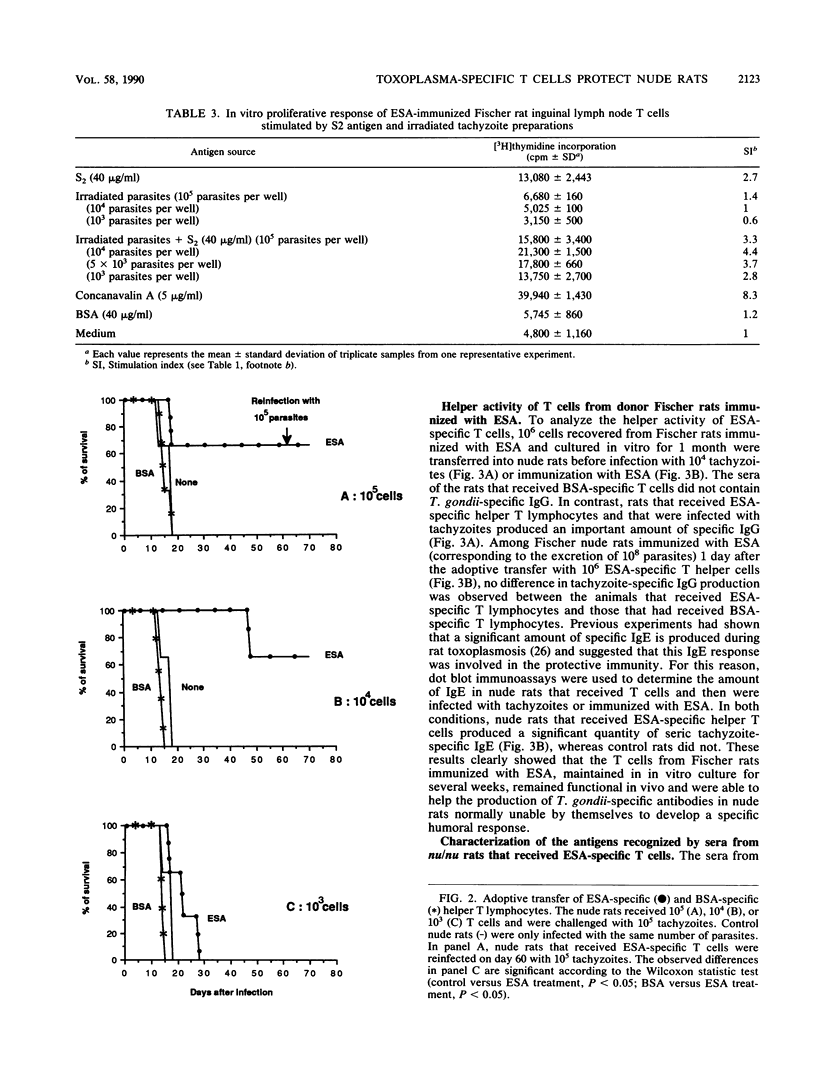
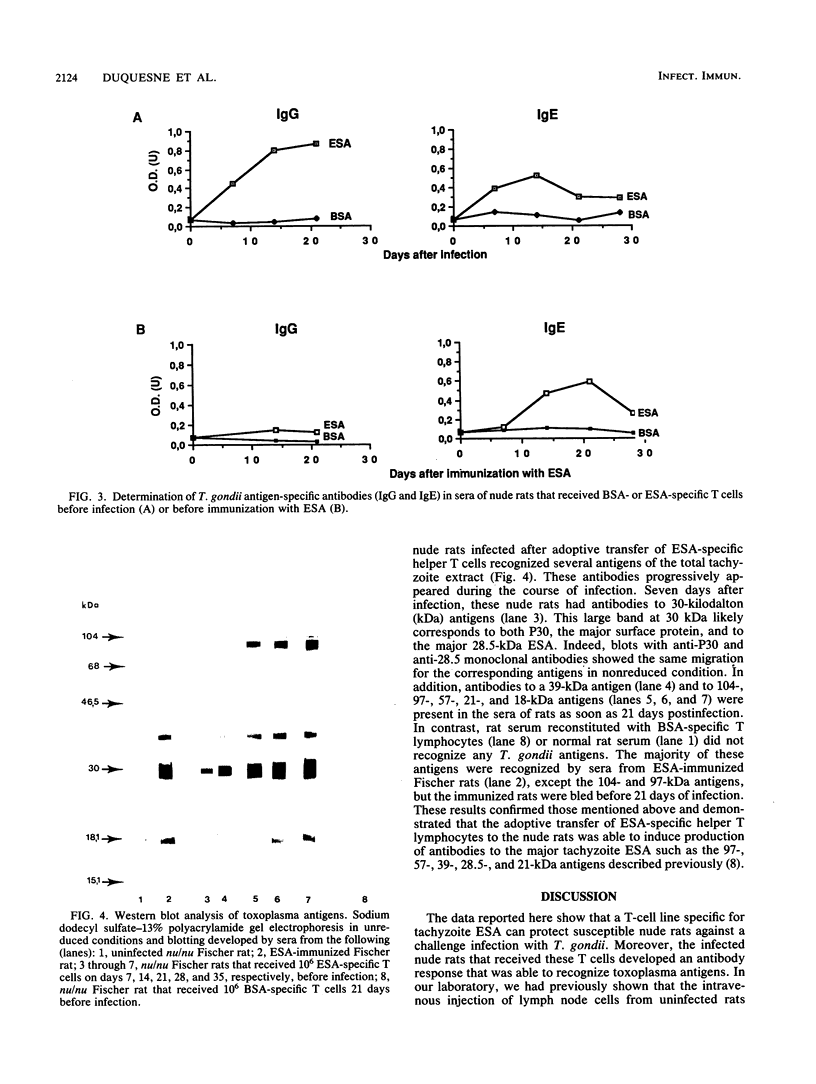
In the present work we demonstrate the implication of excreted-secreted antigens in eliciting the protective cell-mediated immunity developed by rats toward Toxoplasma gondii. We first showed that 10(4) specific T cells from T. gondii-infected rats conferred to nude rats the ability to resist an infection by the highly virulent RH strain of T. gondii. In a second series of experiments, the role of excreted-secreted antigens in this protection was demonstrated. After the adoptive transfer to nude rats of various doses (10(3), 10(4), 10(5)) of excreted-secreted antigen-specific helper T cells (propagated in vitro during one month), significant protection toward T. gondii was induced. Moreover, these cells were responsible for a specific antibody response in nude rats, which are normally unable to develop any specific humoral response. The specificity of these antibodies was directed toward different molecules with molecular masses of 104, 97, 57, 39, 30, 21, and 18 kilodaltons; some of these have been previously characterized as major excreted-secreted antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. E., Hagemo A., Knoblock K., Dubey J. P. Toxoplasma gondii: microassay to differentiate toxoplasma inhibiting factor and interleukin 2. Exp Parasitol. 1983 Jun;55(3):320–330. doi: 10.1016/0014-4894(83)90029-2. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P. Vaccination against parasitic diseases: some alternative concepts for the definition of protective antigens. Ann Inst Pasteur Immunol. 1988 Jan-Feb;139(1):109–117. doi: 10.1016/0769-2625(88)90135-3. [DOI] [PubMed] [Google Scholar]
- Catterall J. R., Sharma S. D., Remington J. S. Oxygen-independent killing by alveolar macrophages. J Exp Med. 1986 May 1;163(5):1113–1131. doi: 10.1084/jem.163.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesbron-Delauw M. F., Guy B., Torpier G., Pierce R. J., Lenzen G., Cesbron J. Y., Charif H., Lepage P., Darcy F., Lecocq J. P. Molecular characterization of a 23-kilodalton major antigen secreted by Toxoplasma gondii. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7537–7541. doi: 10.1073/pnas.86.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla M., Frenkel J. K. Specific mediation of cellular immunity to Toxoplasma gondii in somatic cells of mice. Infect Immun. 1984 Dec;46(3):862–866. doi: 10.1128/iai.46.3.862-866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla M., Guerrero O. M., Solano E. Lack of multiplication of Toxoplasma in macrophages of rats in vitro. J Parasitol. 1982 Oct;68(5):952–955. [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Frenkel J. K. Adoptive immunity to intracellular infection. J Immunol. 1967 Jun;98(6):1309–1319. [PubMed] [Google Scholar]
- Frenkel J. K. Pathophysiology of toxoplasmosis. Parasitol Today. 1988 Oct;4(10):273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- Gemsa D., Debatin K. M., Kramer W., Kubelka C., Deimann W., Kees U., Krammer P. H. Macrophage-activating factors from different T cell clones induce distinct macrophage functions. J Immunol. 1983 Aug;131(2):833–844. [PubMed] [Google Scholar]
- Hughes H. P., Connelly C. A., Strangeways J. E., Hudson L. Antigen specific lymphocyte transformation induced by secreted antigens from Toxoplasma gondii. Clin Exp Immunol. 1984 Dec;58(3):539–547. [PMC free article] [PubMed] [Google Scholar]
- Hughes H. P., van Knapen F. Characterisation of a secretory antigen from Toxoplasma gondii and its role in circulating antigen production. Int J Parasitol. 1982 Oct;12(5):433–437. doi: 10.1016/0020-7519(82)90073-x. [DOI] [PubMed] [Google Scholar]
- Johnson A. M., McDonald P. J., Neoh S. H. Monoclonal antibodies to Toxoplasma cell membrane surface antigens protect mice from toxoplasmosis. J Protozool. 1983 May;30(2):351–356. doi: 10.1111/j.1550-7408.1983.tb02929.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Khan I. A., Smith K. A., Kasper L. H. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J Immunol. 1988 Nov 15;141(10):3600–3605. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg R. E., Frenkel J. K. Toxoplasmosis in nude mice. J Parasitol. 1977 Apr;63(2):219–221. [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. AIDS commentary. Toxoplasmic encephalitis. J Infect Dis. 1988 Jan;157(1):1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S. Protection against experimental toxoplasmosis by adoptive immunotherapy. J Immunol. 1986 Nov 1;137(9):2985–2990. [PubMed] [Google Scholar]
- Ridel P. R., Auriault C., Darcy F., Pierce R. J., Leite P., Santoro F., Neyrinck J. L., Kusnierz J. P., Capron A. Protective role of IgE in immunocompromised rat toxoplasmosis. J Immunol. 1988 Aug 1;141(3):978–983. [PubMed] [Google Scholar]
- Sabin A. B., Feldman H. A. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma). Science. 1948 Dec 10;108(2815):660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- Santoro F., Auriault C., Leite P., Darcy F., Capron A. Infection du rat athymique par Toxoplasma gondii. C R Acad Sci III. 1987;304(11):297–300. [PubMed] [Google Scholar]
- Schreiber R. D., Feldman H. A. Identification of the activator system for antibody to Toxoplasma as the classical complement pathway. J Infect Dis. 1980 Mar;141(3):366–369. doi: 10.1093/infdis/141.3.366. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Araujo F. G., Remington J. S. Toxoplasma antigen isolated by affinity chromatography with monoclonal antibody protects mice against lethal infection with Toxoplasma gondii. J Immunol. 1984 Dec;133(6):2818–2820. [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Production and properties of immune interferon from spleen cell cultures of Toxoplasma-infected mice. Microbiol Immunol. 1980;24(11):1109–1120. doi: 10.1111/j.1348-0421.1980.tb02915.x. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Sharma S. D. Ultrastructural localization of an intracellular Toxoplasma protein that induces protection in mice. Infect Immun. 1987 Sep;55(9):2137–2141. doi: 10.1128/iai.55.9.2137-2141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol. 1988 Jun 1;140(11):3943–3946. [PubMed] [Google Scholar]