Abstract
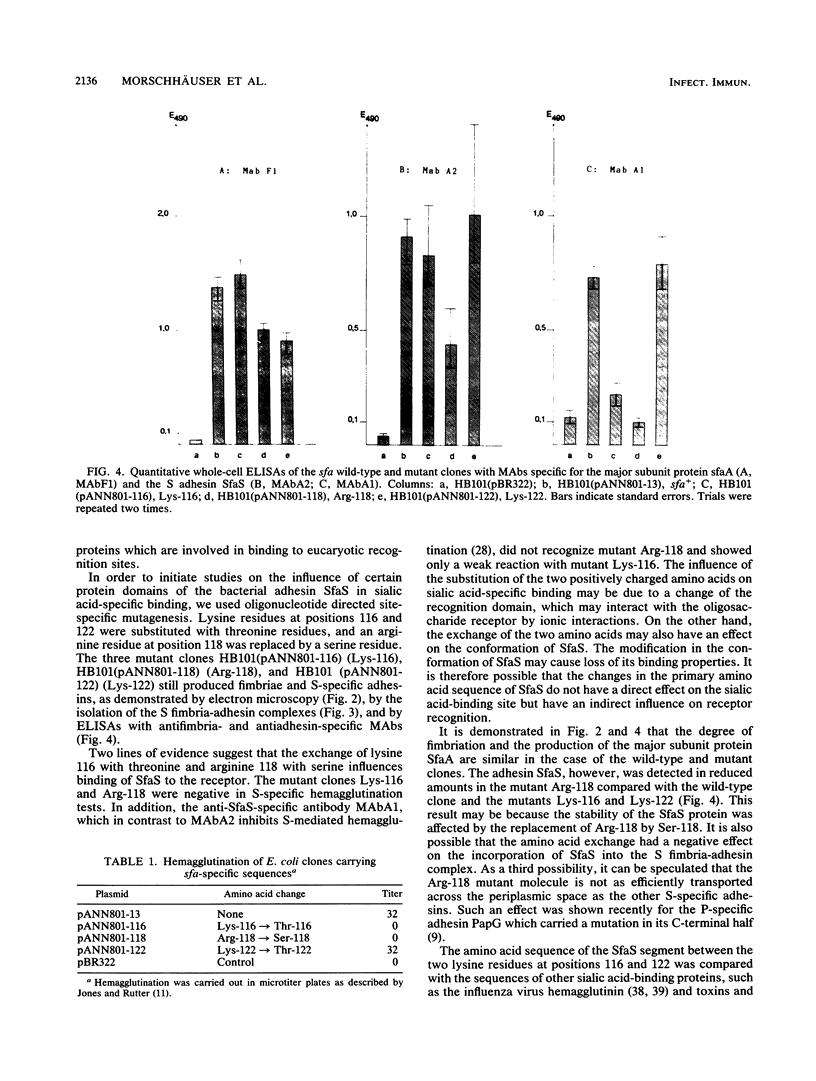
The gene coding for the sialic acid-specific adhesin SfaS produced by the S fimbrial adhesin (sfa) determinant of Escherichia coli has been modified by oligonucleotide-directed, site-specific mutagenesis. Lysine 116, arginine 118, and lysine 122 were replaced by threonine, serine, and threonine, respectively. The mutagenized gene clusters were able to produce S fimbrial adhesin complexes consisting of the S-specific subunit proteins including the adhesin SfaS. The mutant clones were further characterized by hemagglutination and by enzyme-linked immunoassay tests with antifimbria- and anti-adhesin-specific monoclonal antibodies, one of which is able to block S-specific binding (Moch et al., Proc. Natl. Acad. Sci. USA 84:3462-3466, 1987). The lysine-122 mutant clone was indistinguishable from the wild-type clone in these assays. Replacement of lysine 116 and arginine 118, however, abolished hemagglutination and resulted in clones which showed a weak (lysine 116) or a negative (arginine 118) reaction with the antiadhesin-specific antibody A1. We therefore suggest that lysine 116 and arginine 118 have an influence on binding of SfaS to the sialic acid residue of the receptor molecule. Substitution of arginine 118 by serine also had a negative effect on the amount of SfaS adhesin proteins isolated from the S fimbrial adhesin complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan M., Coleman D. C., Smyth C. J. Molecular cloning and characterization of the genetic determinant encoding CS3 fimbriae of enterotoxigenic Escherichia coli. Microb Pathog. 1987 Mar;2(3):195–209. doi: 10.1016/0882-4010(87)90021-0. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Burks M. F., Zupan A., Dallas W. S., Jacob C. O., Ludwig D. S. Epitopes of the cholera family of enterotoxins. Rev Infect Dis. 1987 May-Jun;9(3):544–561. doi: 10.1093/clinids/9.3.544. [DOI] [PubMed] [Google Scholar]
- Fukuta S., Magnani J. L., Twiddy E. M., Holmes R. K., Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988 Jul;56(7):1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J. Genetic determinants coding for fimbriae and adhesins of extraintestinal Escherichia coli. Curr Top Microbiol Immunol. 1990;151:1–27. doi: 10.1007/978-3-642-74703-8_1. [DOI] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoschützky H., Lottspeich F., Jann K. Isolation and characterization of the alpha-galactosyl-1,4-beta-galactosyl-specific adhesin (P adhesin) from fimbriated Escherichia coli. Infect Immun. 1989 Jan;57(1):76–81. doi: 10.1128/iai.57.1.76-81.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Lindberg F., Magnusson G., Kihlberg J., Tennent J. M., Normark S. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4357–4361. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Simons B. H., de Graaf F. K. The role of lysine-132 and arginine-136 in the receptor-binding domain of the K99 fibrillar subunit. EMBO J. 1987 Jun;6(6):1805–1808. doi: 10.1002/j.1460-2075.1987.tb02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- Karjalainen T. K., Evans D. G., So M., Lee C. H. Molecular cloning and nucleotide sequence of the colonization factor antigen I gene of Escherichia coli. Infect Immun. 1989 Apr;57(4):1126–1130. doi: 10.1128/iai.57.4.1126-1130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987 Jul;208(3):439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Klemm P. Primary structure of the CFA1 fimbrial protein from human enterotoxigenic Escherichia coli strains. Eur J Biochem. 1982 May 17;124(2):339–348. doi: 10.1111/j.1432-1033.1982.tb06597.x. [DOI] [PubMed] [Google Scholar]
- Knapp S., Hacker J., Then I., Müller D., Goebel W. Multiple copies of hemolysin genes and associated sequences in the chromosomes of uropathogenic Escherichia coli strains. J Bacteriol. 1984 Sep;159(3):1027–1033. doi: 10.1128/jb.159.3.1027-1033.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J., Vinal A. C., Dallas W. S. Nucleotide sequence comparison between heat-labile toxin B-subunit cistrons from Escherichia coli of human and porcine origin. Infect Immun. 1985 Apr;48(1):73–77. doi: 10.1128/iai.48.1.73-77.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Ludwig D. S., Holmes R. K., Schoolnik G. K. Chemical and immunochemical studies on the receptor binding domain of cholera toxin B subunit. J Biol Chem. 1985 Oct 15;260(23):12528–12534. [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Minion F. C., Abraham S. N., Beachey E. H., Goguen J. D. The genetic determinant of adhesive function in type 1 fimbriae of Escherichia coli is distinct from the gene encoding the fimbrial subunit. J Bacteriol. 1986 Mar;165(3):1033–1036. doi: 10.1128/jb.165.3.1033-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono E., Abe K., Nakazawa M., Naiki M. Ganglioside epitope recognized by K99 fimbriae from enterotoxigenic Escherichia coli. Infect Immun. 1989 Mar;57(3):907–911. doi: 10.1128/iai.57.3.907-911.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985 Dec;95(3):551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Hacker J., Schmoll T., Jarchau T., Korhonen T. K., Goebel W. Analysis of the genetic determinants coding for the S-fimbrial adhesin (sfa) in different Escherichia coli strains causing meningitis or urinary tract infections. Infect Immun. 1986 Dec;54(3):646–653. doi: 10.1128/iai.54.3.646-653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Hoschützky H., Jann K., Van Die I., Hacker J. Gene clusters for S fimbrial adhesin (sfa) and F1C fimbriae (foc) of Escherichia coli: comparative aspects of structure and function. J Bacteriol. 1988 Sep;170(9):3983–3990. doi: 10.1128/jb.170.9.3983-3990.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Hoschützky H., Morschhäuser J., Lottspeich F., Jann K., Hacker J. Analysis of genes coding for the sialic acid-binding adhesin and two other minor fimbrial subunits of the S-fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1989 Dec;3(12):1735–1744. doi: 10.1111/j.1365-2958.1989.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Smit H., Gaastra W., Kamerling J. P., Vliegenthart J. F., de Graaf F. K. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect Immun. 1984 Nov;46(2):578–584. doi: 10.1128/iai.46.2.578-584.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]





