Abstract
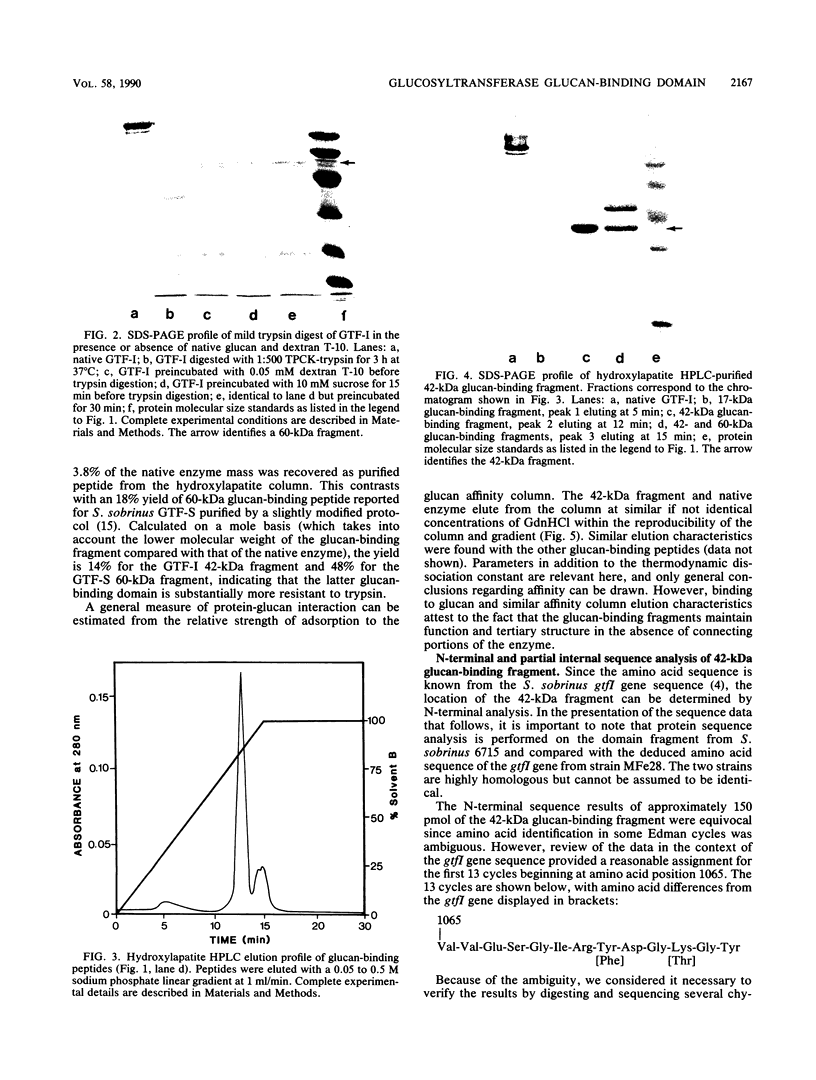
Mild trypsin proteolysis of Streptococcus sobrinus sucrose:3-alpha-D-glucosyltransferase (GTF-I) reduced most of the enzyme to small products but left a few large fragments undigested. The digest had no glucosyl transfer activity, but several digestion products had an affinity for glucan equivalent to that of the native enzyme. The glucan-binding fragments ranged in size from 17 to 60 kilodaltons (kDa), with a particularly prominent 42-kDa fragment. The largest of these (60 kDa) appears to be the full extent of the domain since it increases in abundance when the enzyme is protected with glucan during proteolysis. The presence of smaller fragments with glucan-binding function and intact tertiary structure indicates that the full domain must be built on glucan-binding subdomains. Among the range of glucan-binding fragments, only the 42-kDa segment could be satisfactorily purified. It was subjected to N-terminal sequence analysis and, because of some ambiguity, was also subjected to chymotrypsin digestion and sequence of several chymotryptic peptides. The sequence data established that the 42-kDa domain fragment is initiated approximately two-thirds into the 170-kDa enzyme in a region previously identified as a segment of the gene that includes the glucan-binding domain (J. J. Ferretti, M. L. Gilpin, and R. R. B. Russell, J. Bacteriol. 169:4271-4278, 1987). The site is approximately 60 kDa from the C terminus and covers a region characterized by extensive amino acid sequence repeats. The data are discussed in the context of the size range of the glucan-binding fragments and subdomain architecture of the full glucan-binding domain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banas J. A., Russell R. R., Ferretti J. J. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun. 1990 Mar;58(3):667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. K. Molecular weights of protein multimers from polyacrylamide gel electrophoresis. Anal Biochem. 1977 Apr;78(2):513–519. doi: 10.1016/0003-2697(77)90111-7. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilpin M. L., Russell R. R. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987 Sep;169(9):4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke D. H., Harris D. C., Shively J. E. Microsequence analysis of peptides and proteins. V. Design and performance of a novel gas-liquid-solid phase instrument. Anal Biochem. 1985 Jun;147(2):315–330. doi: 10.1016/0003-2697(85)90278-7. [DOI] [PubMed] [Google Scholar]
- Janin J., Wodak S. J. Structural domains in proteins and their role in the dynamics of protein function. Prog Biophys Mol Biol. 1983;42(1):21–78. doi: 10.1016/0079-6107(83)90003-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Koga K., Hayashida O., Nakano Y., Hasegawa Y. Glucan-binding domain of a glucosyltransferase from Streptococcus sobrinus: isolation of a 55-kilodalton peptide from a trypsin digest of glucosyltransferase prebound to insoluble glucan. Infect Immun. 1989 Jul;57(7):2210–2213. doi: 10.1128/iai.57.7.2210-2213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooser G., Iwaoka K. R. Sucrose 6-alpha-D-glucosyltransferase from Streptococcus sobrinus: characterization of a glucosyl-enzyme complex. Biochemistry. 1989 Jan 24;28(2):443–449. doi: 10.1021/bi00428a006. [DOI] [PubMed] [Google Scholar]
- Mooser G., Shur D., Lyou M., Watanabe C. Kinetic studies on dextransucrase from the cariogenic oral bacterium Streptococcus mutans. J Biol Chem. 1985 Jun 10;260(11):6907–6915. [PubMed] [Google Scholar]
- Mooser G., Wong C. Isolation of a glucan-binding domain of glucosyltransferase (1,6-alpha-glucan synthase) from Streptococcus sobrinus. Infect Immun. 1988 Apr;56(4):880–884. doi: 10.1128/iai.56.4.880-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A. Carbohydrate-binding proteins: tertiary structures and protein-sugar interactions. Annu Rev Biochem. 1986;55:287–315. doi: 10.1146/annurev.bi.55.070186.001443. [DOI] [PubMed] [Google Scholar]
- Schumacher G. F., Schill W. B. Radial diffusion in gel for micro determination of enzymes. II. Plasminogen activator, elastase, and nonspecific proteases. Anal Biochem. 1972 Jul;48(1):9–26. doi: 10.1016/0003-2697(72)90165-0. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Ueda S., Kuramitsu H. K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987 Sep;169(9):4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Shiroza T., Kuramitsu H. K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988 Sep 15;69(1):101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- Wetlaufer D. B. Nucleation, rapid folding, and globular intrachain regions in proteins. Proc Natl Acad Sci U S A. 1973 Mar;70(3):697–701. doi: 10.1073/pnas.70.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]