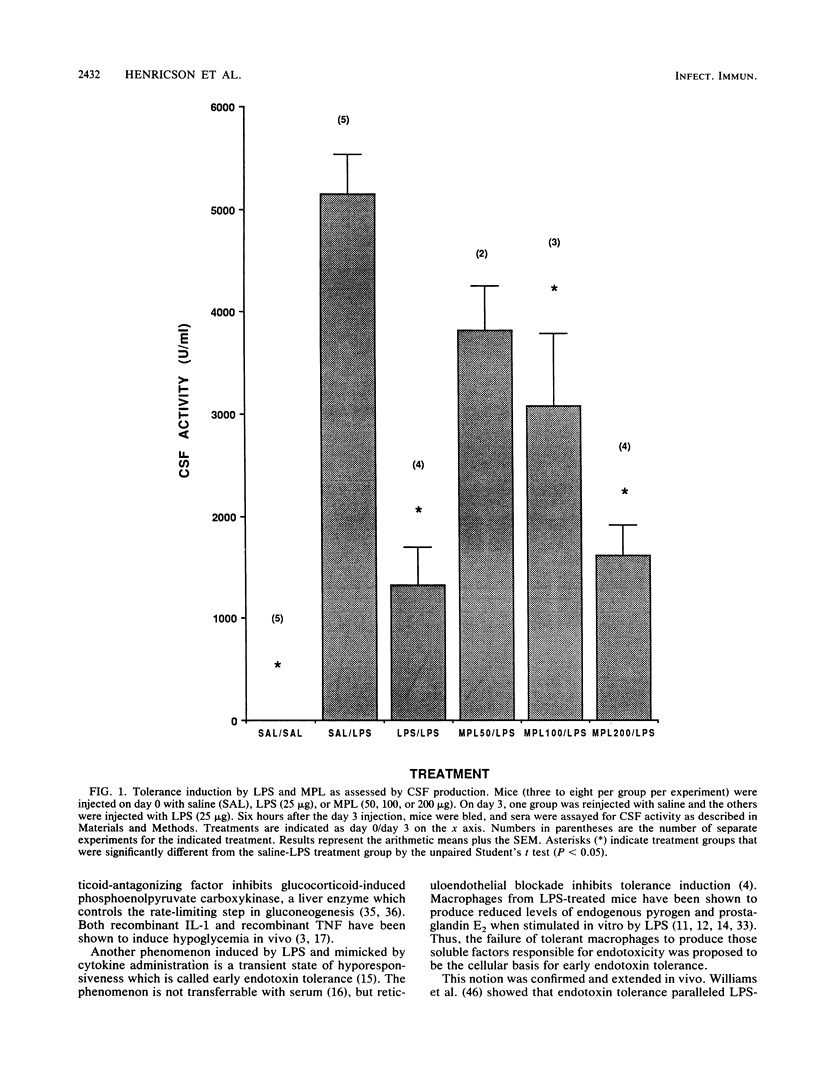
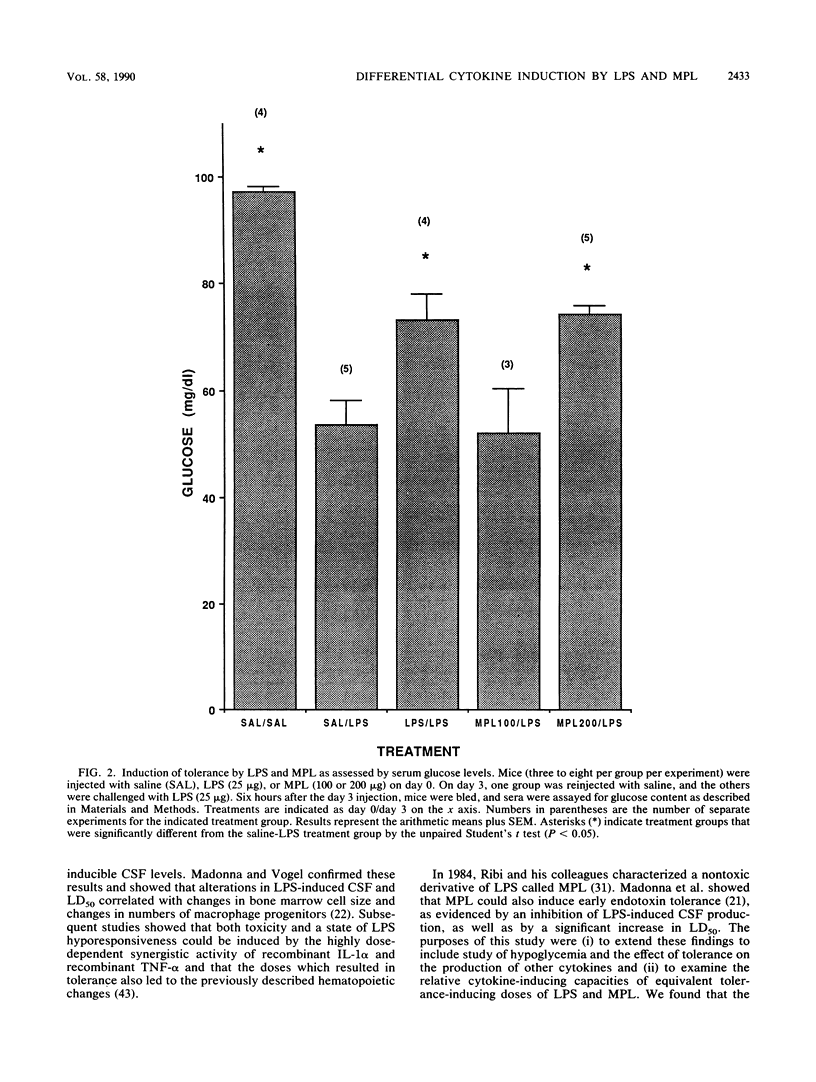
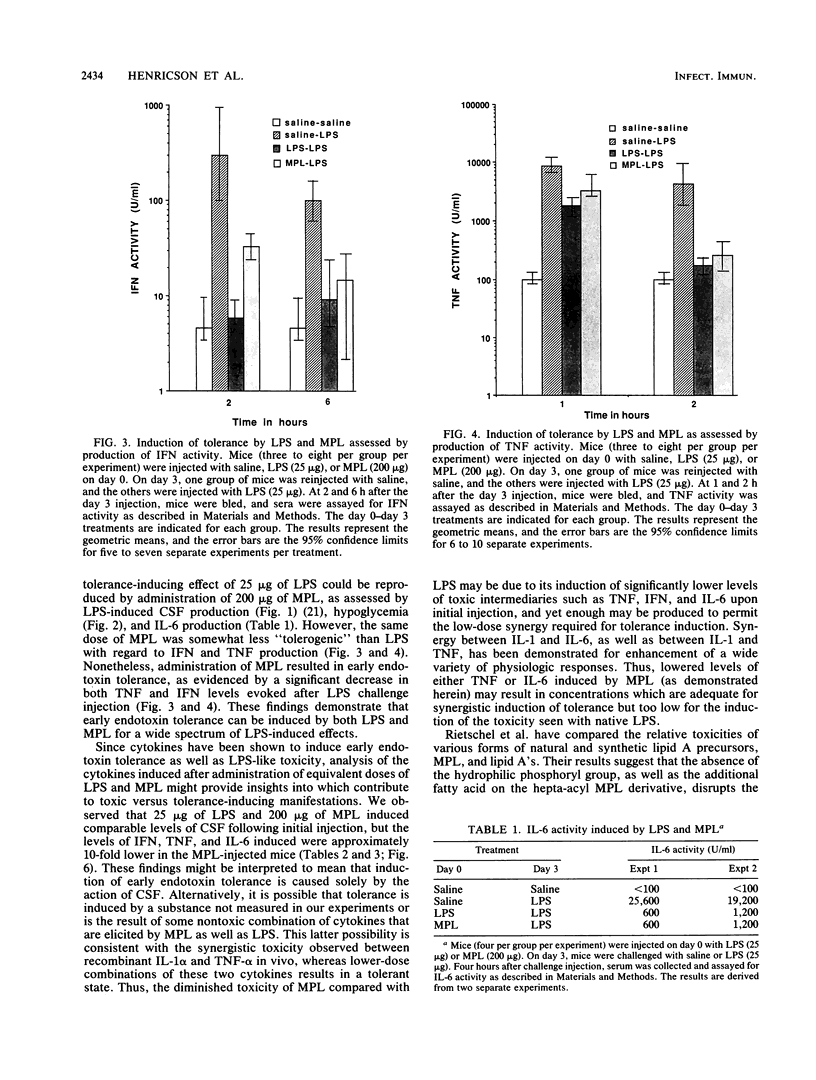
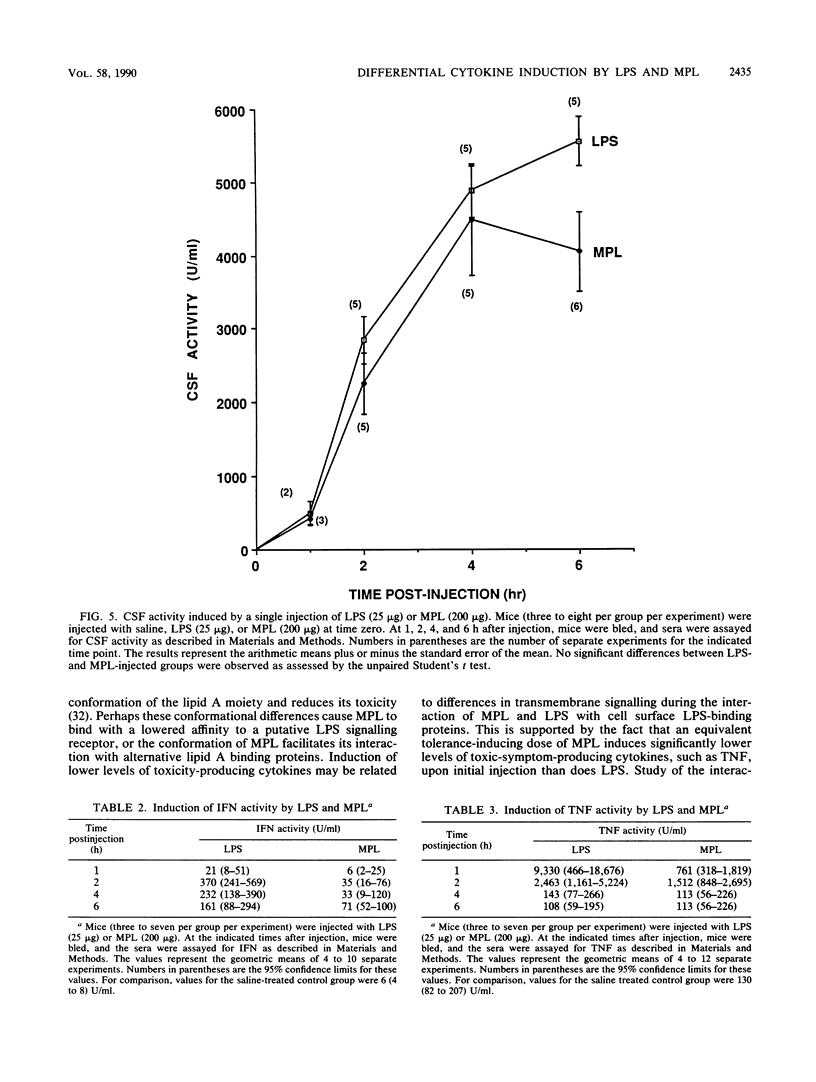
Abstract
The phenomenon of early endotoxin tolerance, which is induced by sublethal injection of lipopolysaccharide (LPS), results in a protracted period of hyporesponsiveness that is most profound at 3 to 4 days after injection and is marked by reduced cytokine production after a challenge injection of LPS. Early endotoxin tolerance is also induced by the nontoxic LPS derivative monophosphoryl lipid A (MPL), although much more of the monophosphoryl derivative is required to produce a state of tolerance equivalent to that evoked by LPS. In this study, equivalent tolerance-inducing doses of LPS and MPL were tested, and the levels of cytokines induced by LPS and MPL were compared. Although induced levels of colony-stimulating factor were comparable following doses of LPS and MPL that elicited an equivalent state of early endotoxin tolerance, levels of tumor necrosis factor, interleukin-6, and interferon were significantly lower in MPL-injected animals. These results suggest that the lowered toxicity of MPL may be related to its elicitation of significantly lower levels of potentially toxic intermediaries such as tumor necrosis factor, interferon, and interleukin-6.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Joslin F. G., Massoni R. J. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J Immunol. 1985 Jun;134(6):3868–3875. [PubMed] [Google Scholar]
- Bauss F., Dröge W., Männel D. N. Tumor necrosis factor mediates endotoxic effects in mice. Infect Immun. 1987 Jul;55(7):1622–1625. doi: 10.1128/iai.55.7.1622-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry L. J. Bacterial toxins. CRC Crit Rev Toxicol. 1977 Nov;5(3):239–318. doi: 10.3109/10408447709082601. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Brade H., Brade L., Schade U., Zähringer U., Holst O., Kuhn H. M., Rozalski A., Röhrscheidt E., Rietschel E. T. Structure, endotoxicity, immunogenicity and antigenicity of bacterial lipopolysaccharides (endotoxins, O-antigens). Prog Clin Biol Res. 1988;272:17–45. [PubMed] [Google Scholar]
- Dinarello C. A., Bernheim H. A. Ability of human leukocytic pyrogen to stimulate brain prostaglandin synthesis in vitro. J Neurochem. 1981 Sep;37(3):702–708. doi: 10.1111/j.1471-4159.1982.tb12544.x. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Bodel P. T., Atkins E. The role of the liver in the production of fever and in pyrogenic tolerance. Trans Assoc Am Physicians. 1968;81:334–344. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. Pathogenesis of fever in man. N Engl J Med. 1978 Mar 16;298(11):607–612. doi: 10.1056/NEJM197803162981107. [DOI] [PubMed] [Google Scholar]
- Favorite G. O., Morgan H. R. EFFECTS PRODUCED BY THE INTRAVENOUS INJECTION IN MAN OF A TOXIC ANTIGENIC MATERIAL DERIVED FROM EBERTHELLA TYPHOSA: CLINICAL, HEMATOLOGICAL, CHEMICAL AND SEROLOGICAL STUDIES. J Clin Invest. 1942 Sep;21(5):589–599. doi: 10.1172/JCI101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREISMAN S. E., WOODWARD W. E. MECHANISMS OF ENDOTOXIN TOLERANCE. 3. THE REFRACTORY STATE DURING CONTINUOUS INTRAVENOUS INFUSIONS OF ENDOTOXIN. J Exp Med. 1965 Jun 1;121:911–933. doi: 10.1084/jem.121.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. R., Stith R. D., McCallum R. E. Interleukin 1: a regulatory role in glucocorticoid-regulated hepatic metabolism. J Immunol. 1986 Aug 1;137(3):858–862. [PubMed] [Google Scholar]
- Hogan M. M., Vogel S. N. Production of tumor necrosis factor by rIFN-gamma-primed C3H/HeJ (Lpsd) macrophages requires the presence of lipid A-associated proteins. J Immunol. 1988 Dec 15;141(12):4196–4202. [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Madonna G. S., Peterson J. E., Ribi E. E., Vogel S. N. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986 Apr;52(1):6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna G. S., Vogel S. N. Early endotoxin tolerance is associated with alterations in bone marrow-derived macrophage precursor pools. J Immunol. 1985 Dec;135(6):3763–3771. [PubMed] [Google Scholar]
- Madonna G. S., Vogel S. N. Induction of early-phase endotoxin tolerance in athymic (nude) mice, B-cell-deficient (xid) mice, and splenectomized mice. Infect Immun. 1986 Sep;53(3):707–710. doi: 10.1128/iai.53.3.707-710.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Taguchi M., Kovacs E. J., Young H. A., Oppenheim J. J. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986 Apr 15;136(8):2883–2891. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Moore R. N., Goodrum K. J., Berry L. J. Mediation of an endotoxic effect by macrophages. J Reticuloendothel Soc. 1976 Mar;19(3):187–197. [PubMed] [Google Scholar]
- Raetz C. R., Purcell S., Takayama K. Molecular requirements for B-lymphocyte activation by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4624–4628. doi: 10.1073/pnas.80.15.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E. Beneficial modification of the endotoxin molecule. J Biol Response Mod. 1984;3(1):1–9. [PubMed] [Google Scholar]
- Rietschel E. T., Brade H., Brade L., Brandenburg K., Schade U., Seydel U., Zähringer U., Galanos C., Lüderitz O., Westphal O. Lipid A, the endotoxic center of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Prog Clin Biol Res. 1987;231:25–53. [PubMed] [Google Scholar]
- Rietschel E. T., Schade U., Jensen M., Wollenweber H. W., Lüderitz O., Greisman S. G. Bacterial endotoxins: chemical structure, biological activity and role in septicaemia. Scand J Infect Dis Suppl. 1982;31:8–21. [PubMed] [Google Scholar]
- Rippe D. F., Berry L. J. Immunological quantitation of hepatic tryptophan oxygenase in endotoxin-poisoned mice. Infect Immun. 1973 Oct;8(4):534–539. doi: 10.1128/iai.8.4.534-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe D. F., Berry L. J. Study of inhibition of induction of phosphoenolpyruvate carboxykinase by endotoxin with radial immunodiffusion. Infect Immun. 1972 Nov;6(5):766–772. doi: 10.1128/iai.6.5.766-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S., Familletti P. C., Pestka S. Convenient assay for interferons. J Virol. 1981 Feb;37(2):755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Raetz C. R., Ribi E., Peterson J., Cantrell J. L., Pearson F. C., Wiggins J., Johnson A. G. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect Immun. 1984 Aug;45(2):350–355. doi: 10.1128/iai.45.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Douches S. D., Kaufman E. N., Neta R. Induction of colony stimulating factor in vivo by recombinant interleukin 1 alpha and recombinant tumor necrosis factor alpha 1. J Immunol. 1987 Apr 1;138(7):2143–2148. [PubMed] [Google Scholar]
- Vogel S. N., Kaufman E. N., Tate M. D., Neta R. Recombinant interleukin-1 alpha and recombinant tumor necrosis factor alpha synergize in vivo to induce early endotoxin tolerance and associated hematopoietic changes. Infect Immun. 1988 Oct;56(10):2650–2657. doi: 10.1128/iai.56.10.2650-2657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Williams Z., Hertogs C. F., Pluznik D. H. Use of mice tolerant to lipopolysaccharide to demonstrate requirement of cooperation between macrophages and lymphocytes to generate lipopolysaccharide-induced colony-stimulating factor in vivo. Infect Immun. 1983 Jul;41(1):1–5. doi: 10.1128/iai.41.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


