Abstract
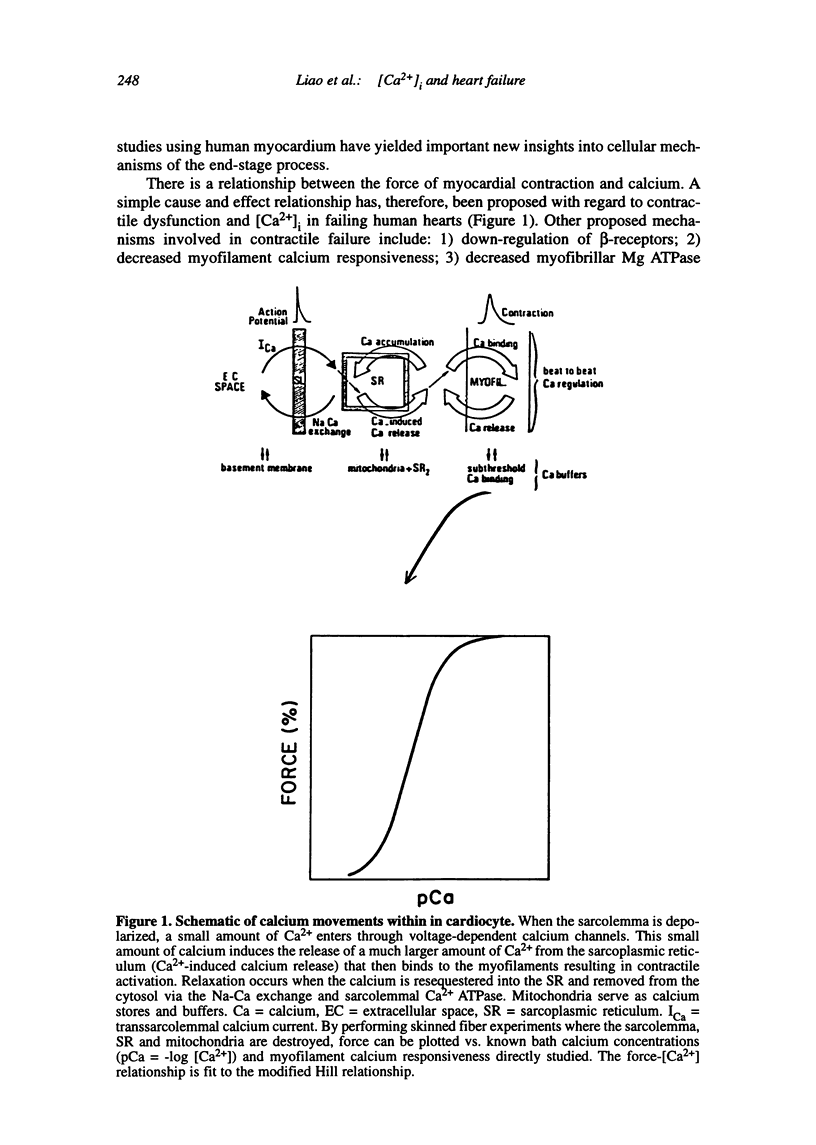
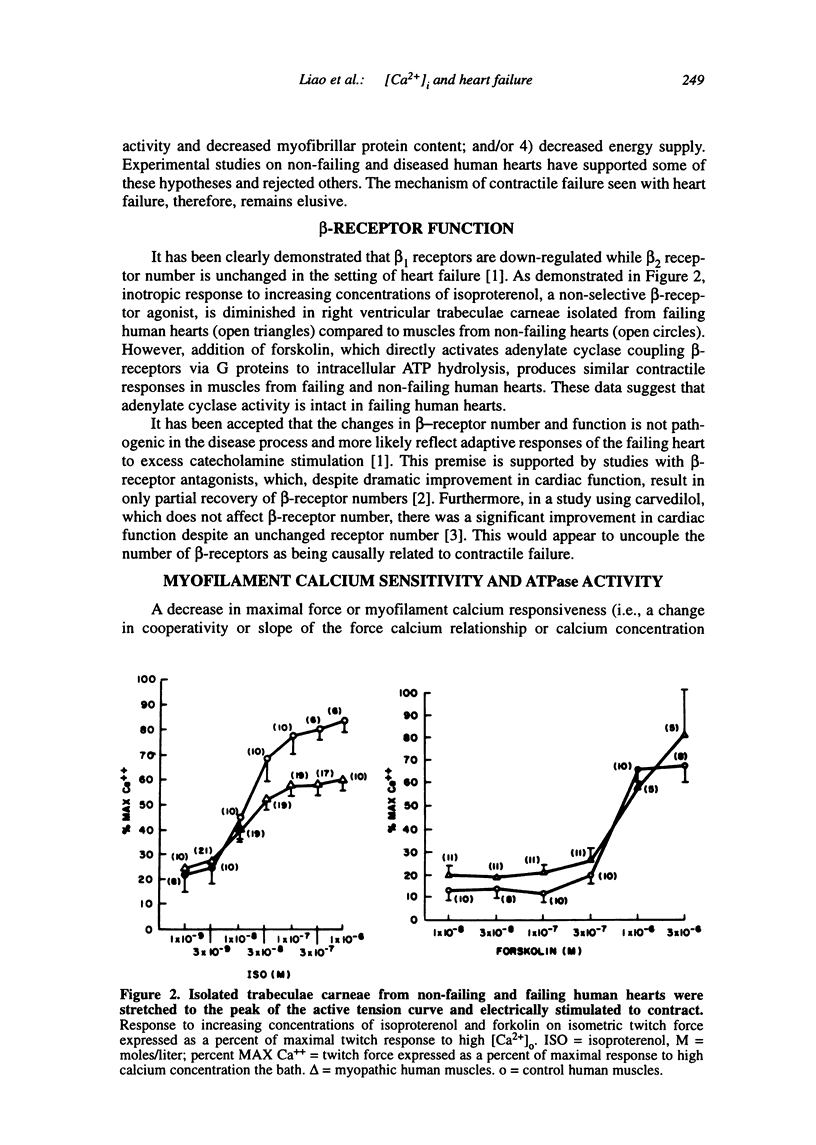
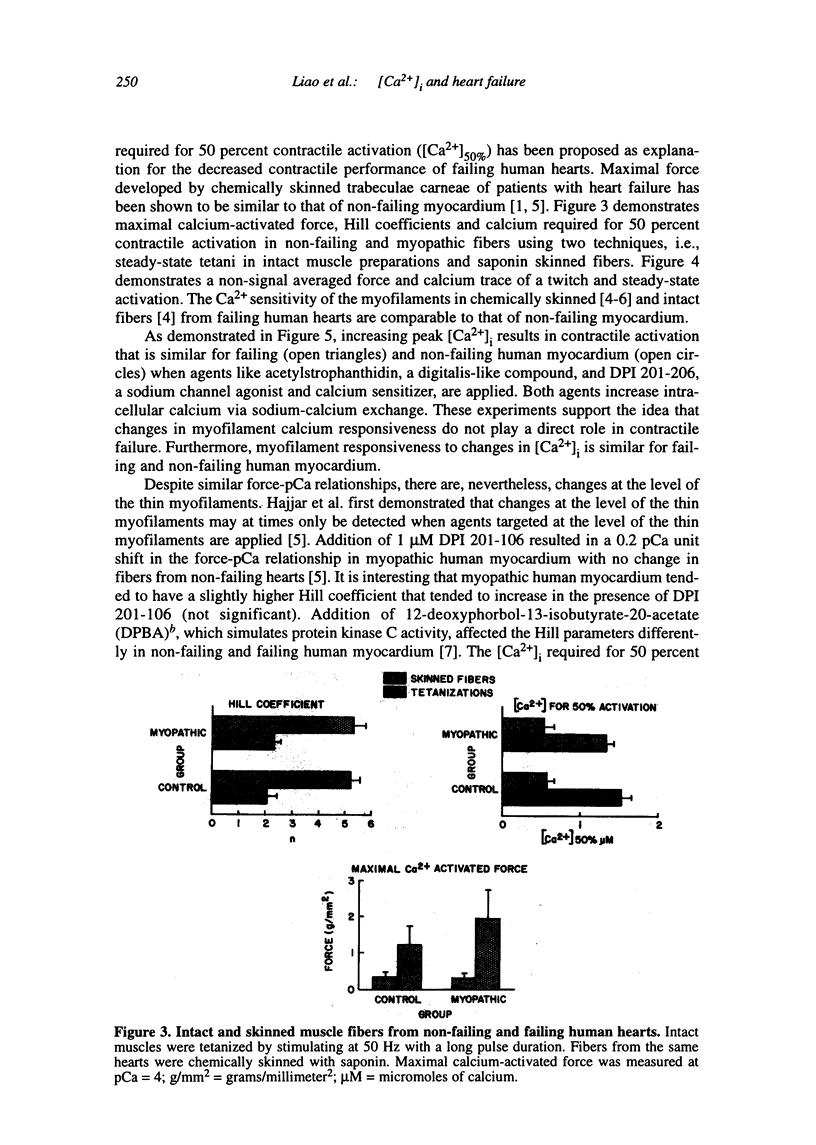
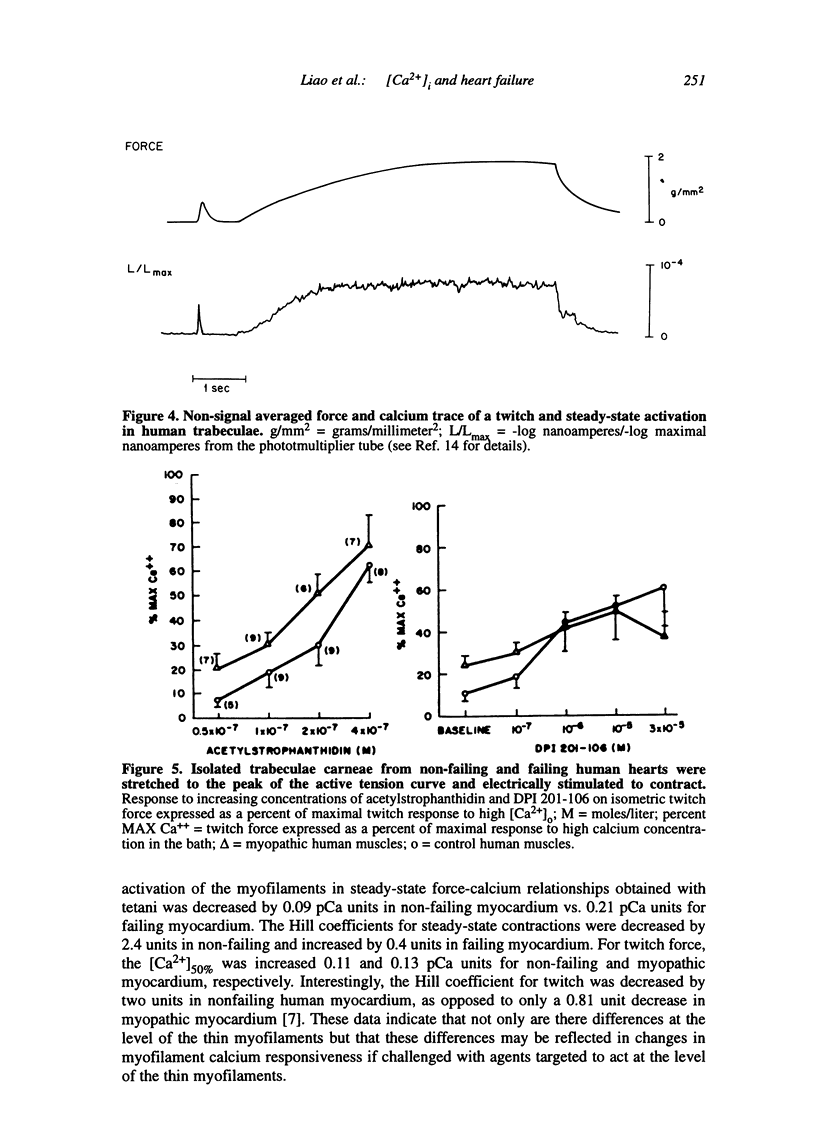
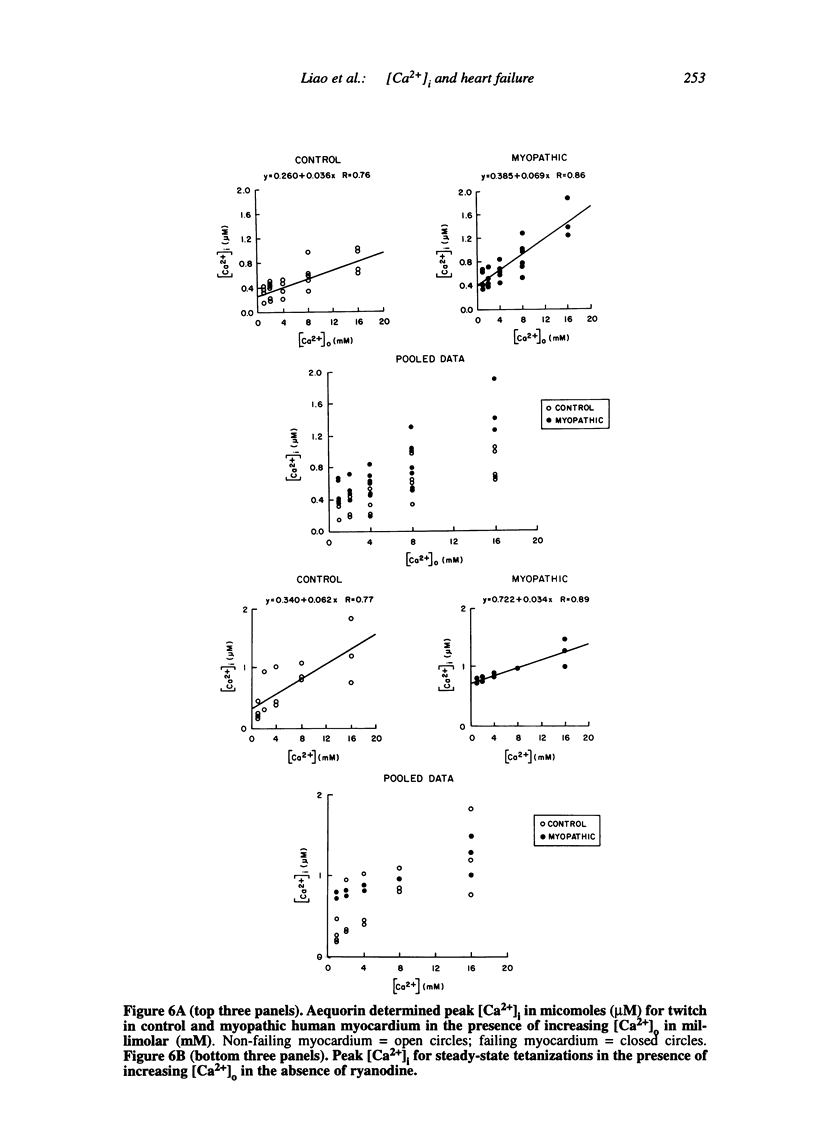
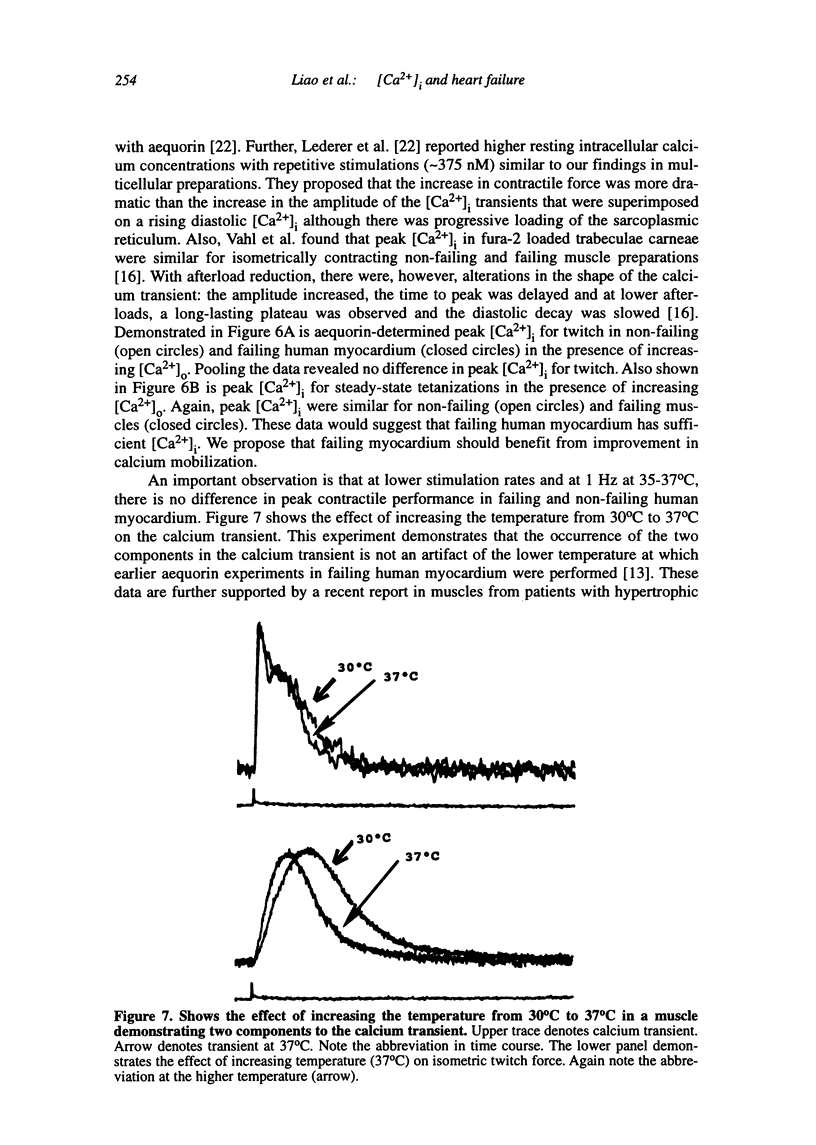
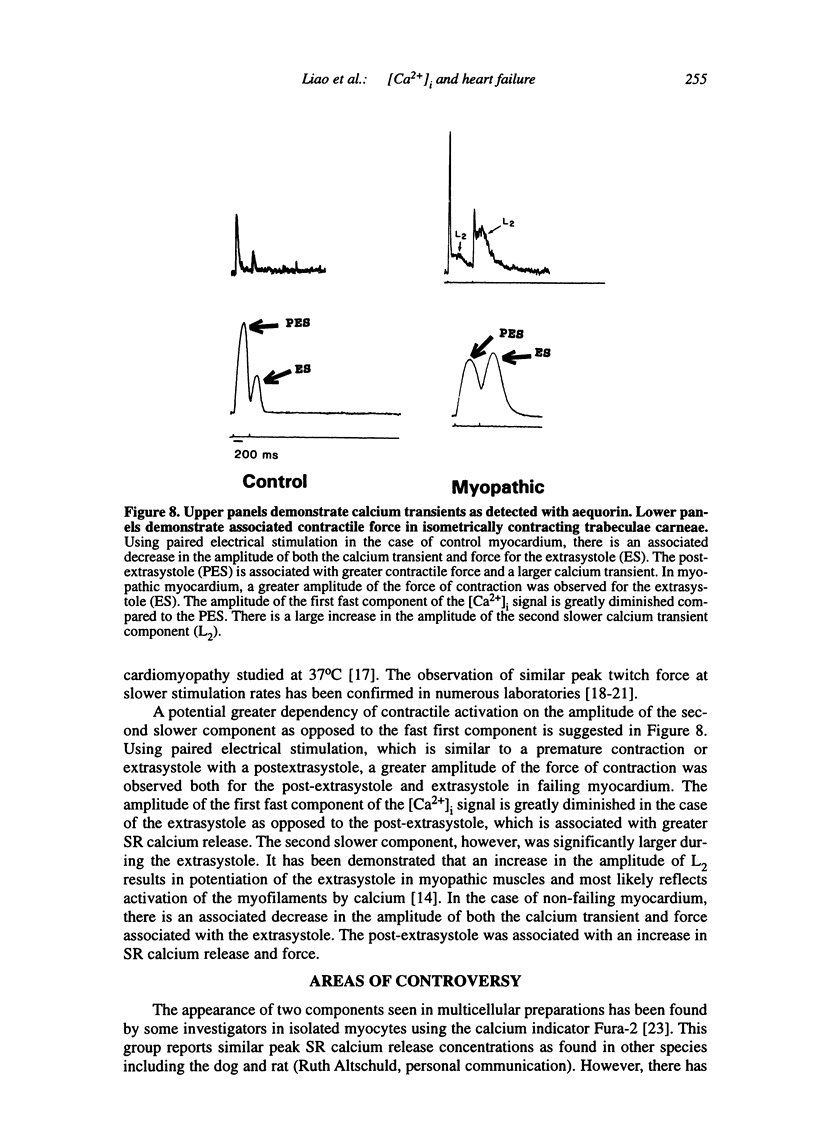
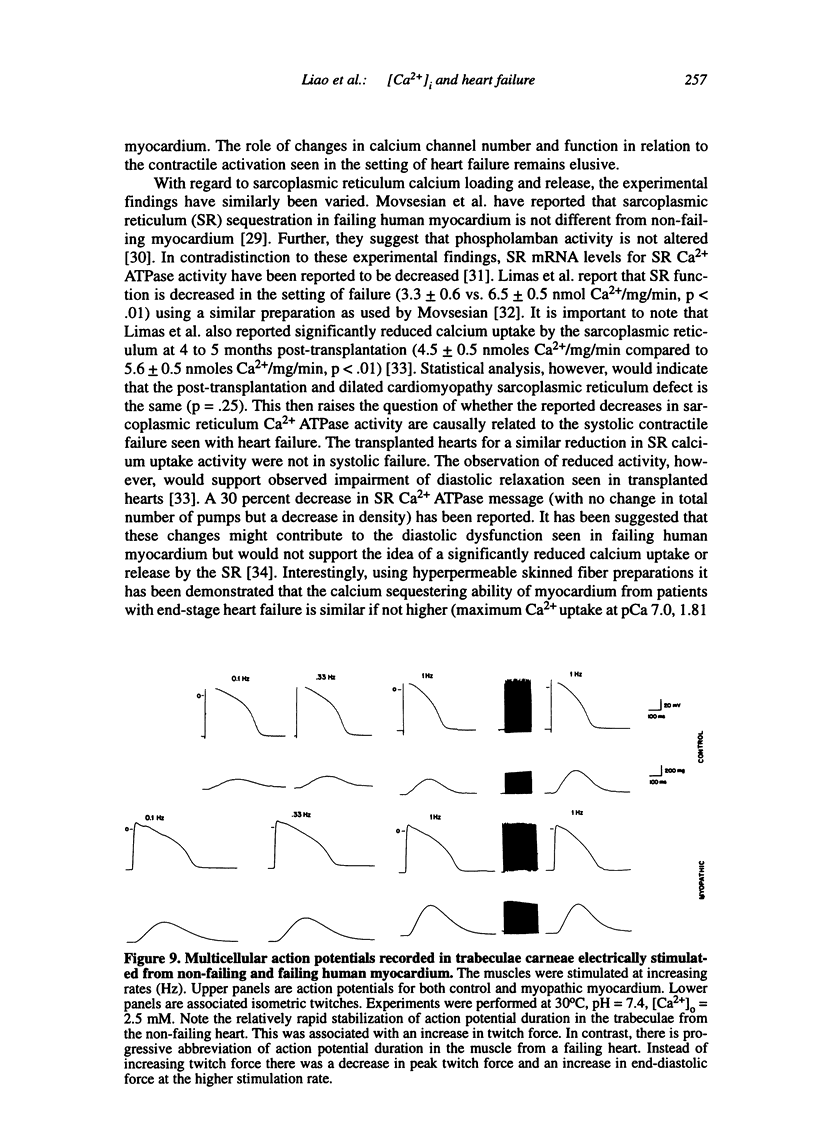
Multiple abnormalities have been reported in the setting of human heart failure. It is unclear whether detected changes reflect adaptive alterations in myocardium subjected to increased and sustained hemodynamic overload or are pathogenic to the disease process. As a result of the observation that the primary defect in heart failure is decreased pump function, investigators have concentrated their efforts on determining systolic [Ca2+]i as a logical corollary and a causative mechanism for contractile dysfunction. A simple cause and effect relationship has therefore been proposed with regard to contractile dysfunction and [Ca2+]i. Yet some investigators have found no difference in peak systolic [Ca2+]i between failing and non-failing human myocardium, whereas others have found peak [Ca2+]i to be significantly reduced in failing hearts. Resting calcium concentrations have been reported either to be elevated in failing human myocardium or not different from non-failing human myocardium. Investigators should now appreciate that the force-calcium relationship is not a simple relationship. One must take into account the prolonged time course and slowed mobilization of [Ca2+]i as opposed to simply peak [Ca2+]i. When put in perspective of mechanisms and determinants of the Ca(2+)-force relationship, we begin to realize that failing human myocardium has the "potential" to generate normal levels of force. Only when stressed by [Ca2+]i overload and/or frequency perturbation does myocardium from patients with end-stage heart disease demonstrate contractile failure. Although [Ca2+]i availability and mobilization are likely to play a role in the systolic as well as diastolic dysfunction reported in human heart failure, it is likely that other mechanisms are involved as well (e.g., myocardial energetics). Myocardial energetics is directly related to [Ca2+]i and mobilization in failing human myocardium, because metabolites, e.g., ADP, inhibit pumps, such as sarcoplasmic reticulum Ca2+ ATPase activity. We therefore conclude that there is a role for intracellular calcium mobilization and myocardial energetics for systolic and diastolic dysfunction seen in human heart failure.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. A., Malouf N. N., Oakeley A. E., Pagani E. D., Allen P. D. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res. 1991 Nov;69(5):1226–1233. doi: 10.1161/01.res.69.5.1226. [DOI] [PubMed] [Google Scholar]
- Arai M., Matsui H., Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res. 1994 Apr;74(4):555–564. doi: 10.1161/01.res.74.4.555. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Characteristics of calcium-current in isolated human ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol. 1991 Aug;23(8):929–937. doi: 10.1016/0022-2828(91)90135-9. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992 Mar;85(3):1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Umans V., Fowler M., Minobe W., Rasmussen R., Zera P., Menlove R., Shah P., Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986 Sep;59(3):297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- Böhm M., Diet F., Kemkes B., Wankerl M., Erdmann E. Inotropic response to DPI 201-106 in the failing human heart. Br J Pharmacol. 1989 Sep;98(1):275–283. doi: 10.1111/j.1476-5381.1989.tb16892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M., Morano I., Pieske B., Rüegg J. C., Wankerl M., Zimmermann R., Erdmann E. Contribution of cAMP-phosphodiesterase inhibition and sensitization of the contractile proteins for calcium to the inotropic effect of pimobendan in the failing human myocardium. Circ Res. 1991 Mar;68(3):689–701. doi: 10.1161/01.res.68.3.689. [DOI] [PubMed] [Google Scholar]
- Chapados R. A., Gruver E. J., Ingwall J. S., Marsh J. D., Gwathmey J. K. Chronic administration of cardiovascular drugs: altered energetics and transmembrane signaling. Am J Physiol. 1992 Nov;263(5 Pt 2):H1576–H1586. doi: 10.1152/ajpheart.1992.263.5.H1576. [DOI] [PubMed] [Google Scholar]
- D'Agnolo A., Luciani G. B., Mazzucco A., Gallucci V., Salviati G. Contractile properties and Ca2+ release activity of the sarcoplasmic reticulum in dilated cardiomyopathy. Circulation. 1992 Feb;85(2):518–525. doi: 10.1161/01.cir.85.2.518. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Patterson R. E., Roberts W. C., Smith T. D., Keiser H. R. Calcium channel binding characteristics in the human heart. Am J Cardiol. 1988 Dec 1;62(17):1281–1284. doi: 10.1016/0002-9149(88)90274-3. [DOI] [PubMed] [Google Scholar]
- Gruver E. J., Glass M. G., Marsh J. D., Gwathmey J. K. An animal model of dilated cardiomyopathy: characterization of dihydropyridine receptors and contractile performance. Am J Physiol. 1993 Nov;265(5 Pt 2):H1704–H1711. doi: 10.1152/ajpheart.1993.265.5.H1704. [DOI] [PubMed] [Google Scholar]
- Gruver E. J., Morgan J. P., Stambler B. S., Gwathmey J. K. Uniformity of calcium channel number and isometric contraction in human right and left ventricular myocardium. Basic Res Cardiol. 1994 Mar-Apr;89(2):139–148. doi: 10.1007/BF00788733. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Copelas L., MacKinnon R., Schoen F. J., Feldman M. D., Grossman W., Morgan J. P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987 Jul;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Hajjar R. J. Effect of protein kinase C activation on sarcoplasmic reticulum function and apparent myofibrillar Ca2+ sensitivity in intact and skinned muscles from normal and diseased human myocardium. Circ Res. 1990 Sep;67(3):744–752. doi: 10.1161/01.res.67.3.744. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Hajjar R. J. Intracellular calcium related to force development in twitch contraction of mammalian myocardium. Cell Calcium. 1990 Sep;11(8):531–538. doi: 10.1016/0143-4160(90)90029-t. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Hajjar R. J. Relation between steady-state force and intracellular [Ca2+] in intact human myocardium. Index of myofibrillar responsiveness to Ca2+. Circulation. 1990 Oct;82(4):1266–1278. doi: 10.1161/01.cir.82.4.1266. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Slawsky M. T., Hajjar R. J., Briggs G. M., Morgan J. P. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest. 1990 May;85(5):1599–1613. doi: 10.1172/JCI114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey J. K., Warren S. E., Briggs G. M., Copelas L., Feldman M. D., Phillips P. J., Callahan M., Jr, Schoen F. J., Grossman W., Morgan J. P. Diastolic dysfunction in hypertrophic cardiomyopathy. Effect on active force generation during systole. J Clin Invest. 1991 Mar;87(3):1023–1031. doi: 10.1172/JCI115061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar R. J., Gwathmey J. K., Briggs G. M., Morgan J. P. Differential effect of DPI 201-106 on the sensitivity of the myofilaments to Ca2+ in intact and skinned trabeculae from control and myopathic human hearts. J Clin Invest. 1988 Nov;82(5):1578–1584. doi: 10.1172/JCI113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar R. J., Gwathmey J. K. Calcium-sensitizing inotropic agents in the treatment of heart failure: a critical view. Cardiovasc Drugs Ther. 1991 Dec;5(6):961–965. doi: 10.1007/BF00143520. [DOI] [PubMed] [Google Scholar]
- Hajjar R. J., Gwathmey J. K. Cross-bridge dynamics in human ventricular myocardium. Regulation of contractility in the failing heart. Circulation. 1992 Dec;86(6):1819–1826. doi: 10.1161/01.cir.86.6.1819. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G., Mulieri L. A., Blanchard E. M., Holubarsch C., Leavitt B. J., Ittleman F., Alpert N. R. Energetics of isometric force development in control and volume-overload human myocardium. Comparison with animal species. Circ Res. 1991 Mar;68(3):836–846. doi: 10.1161/01.res.68.3.836. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G., Mulieri L. A., Leavitt B. J., Allen P. D., Haeberle J. R., Alpert N. R. Alteration of contractile function and excitation-contraction coupling in dilated cardiomyopathy. Circ Res. 1992 Jun;70(6):1225–1232. doi: 10.1161/01.res.70.6.1225. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Berlin J. R., Cohen N. M., Hadley R. W., Bers D. M., Cannell M. B. Excitation-contraction coupling in heart cells. Roles of the sodium-calcium exchange, the calcium current, and the sarcoplasmic reticulum. Ann N Y Acad Sci. 1990;588:190–206. doi: 10.1111/j.1749-6632.1990.tb13210.x. [DOI] [PubMed] [Google Scholar]
- Li Q., Biagi B., Hohl C., Starling R., Stokes B., Altschuld R. Effects of isoproterenol and caffeine on calcium transients and action potentials in human ventricular cardiomyocytes. Prog Clin Biol Res. 1990;327:743–750. [PubMed] [Google Scholar]
- Limas C. J., Olivari M. T., Benditt D. G., Almquist A. Altered calcium uptake by the sarcoplasmic reticulum following cardiac transplantation in humans. Can J Cardiol. 1987 Jun-Aug;3(5):215–219. [PubMed] [Google Scholar]
- Limas C. J., Olivari M. T., Goldenberg I. F., Levine T. B., Benditt D. G., Simon A. Calcium uptake by cardiac sarcoplasmic reticulum in human dilated cardiomyopathy. Cardiovasc Res. 1987 Aug;21(8):601–605. doi: 10.1093/cvr/21.8.601. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Lompré A. M., Duc P., Boheler K. R., Fraysse J. B., Wisnewsky C., Allen P. D., Komajda M., Schwartz K. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 1990 Jan;85(1):305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesian M. A., Bristow M. R., Krall J. Ca2+ uptake by cardiac sarcoplasmic reticulum from patients with idiopathic dilated cardiomyopathy. Circ Res. 1989 Oct;65(4):1141–1144. doi: 10.1161/01.res.65.4.1141. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Colyer J., Wang J. H., Krall J. Phospholamban-mediated stimulation of Ca2+ uptake in sarcoplasmic reticulum from normal and failing hearts. J Clin Invest. 1990 May;85(5):1698–1702. doi: 10.1172/JCI114623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulieri L. A., Hasenfuss G., Leavitt B., Allen P. D., Alpert N. R. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992 May;85(5):1743–1750. doi: 10.1161/01.cir.85.5.1743. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Sugiyama S., Hattori K., Ito T., Ohno K., Takahashi A., Sato W., Takada G., Mayumi B. Multiple mitochondrial DNA deletions exist in cardiomyocytes of patients with hypertrophic or dilated cardiomyopathy. Biochem Biophys Res Commun. 1990 Jul 31;170(2):830–836. doi: 10.1016/0006-291x(90)92166-w. [DOI] [PubMed] [Google Scholar]
- Pagani E. D., Alousi A. A., Grant A. M., Older T. M., Dziuban S. W., Jr, Allen P. D. Changes in myofibrillar content and Mg-ATPase activity in ventricular tissues from patients with heart failure caused by coronary artery disease, cardiomyopathy, or mitral valve insufficiency. Circ Res. 1988 Aug;63(2):380–385. doi: 10.1161/01.res.63.2.380. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. P., Minobe W., Bristow M. R. Calcium antagonist binding sites in failing and nonfailing human ventricular myocardium. Biochem Pharmacol. 1990 Feb 15;39(4):691–696. doi: 10.1016/0006-2952(90)90147-d. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Wodak A. D., Atkinson L., Saunders J. B., Jewitt D. E. Relation between alcohol intake, myocardial enzyme activity, and myocardial function in dilated cardiomyopathy. Evidence for the concept of alcohol induced heart muscle disease. Br Heart J. 1986 Aug;56(2):165–170. doi: 10.1136/hrt.56.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talo A., Stern M. D., Spurgeon H. A., Isenberg G., Lakatta E. G. Sustained subthreshold-for-twitch depolarization in rat single ventricular myocytes causes sustained calcium channel activation and sarcoplasmic reticulum calcium release. J Gen Physiol. 1990 Nov;96(5):1085–1103. doi: 10.1085/jgp.96.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl C. F., Bonz A., Timek T., Hagl S. Intracellular calcium transient of working human myocardium of seven patients transplanted for congestive heart failure. Circ Res. 1994 May;74(5):952–958. doi: 10.1161/01.res.74.5.952. [DOI] [PubMed] [Google Scholar]
- Waagstein F., Caidahl K., Wallentin I., Bergh C. H., Hjalmarson A. Long-term beta-blockade in dilated cardiomyopathy. Effects of short- and long-term metoprolol treatment followed by withdrawal and readministration of metoprolol. Circulation. 1989 Sep;80(3):551–563. doi: 10.1161/01.cir.80.3.551. [DOI] [PubMed] [Google Scholar]
- Wasserman K. Reduced aerobic enzyme activity in skeletal muscles of patients with heart failure. A primary defect or a result of limited cardiac output? Circulation. 1991 Oct;84(4):1868–1870. doi: 10.1161/01.cir.84.4.1868. [DOI] [PubMed] [Google Scholar]


