Abstract
Proteus mirabilis, a common agent of nosocomially acquired and catheter-associated urinary tract infection, is the most frequent cause of infection-induced bladder and kidney stones. Urease-catalyzed urea hydrolysis initiates stone formation in urine and can be inhibited by acetohydroxamic acid and other structural analogs of urea. Since P. mirabilis urease is inducible with urea, there has been some concern that urease inhibitors actually induce urease during an active infection, thus compounding the problem of elevated enzyme activity. Quantitating induction by compounds that simultaneously inhibit urease activity has been difficult. Therefore, to study these problems, we constructed a fusion of ureA (a urease subunit gene) and lacZ (the beta-galactosidase gene) within plasmid pMID1010, which encodes an inducible urease of P. mirabilis expressed in E. coli JM103 (Lac-). The fusion protein, predicted to be 117 kDa, was induced by urea and detected on Western blots (immunoblots) with anti-beta-galactosidase antiserum. Peak beta-galactosidase activity of 9.9 mumol of ONPG (o-nitrophenyl-beta-D-galactopyranoside) hydrolyzed per min per mg of protein, quantitated spectrophotometrically, was induced at 200 mM urea. The uninduced rate was 0.2 mumol of ONPG hydrolyzed per min per mg of protein. Induction was specific for urea, as no structural analog of urea (including acetohydroxamic acid, hydroxyurea, thiourea, hippuric acid, flurofamide, or hydroxylamine) induced fusion protein activity. These data suggest that induction by inactivation of UreR, the urease repressor protein that governs regulation of the urease operon, is specific for urea and does not respond to closely related structural analogs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon N. E., Riddles P. W., Gazzola C., Blakeley R. L., Zerner B. Jack bean urease (EC 3.5.1.5). V. On the mechanism of action of urease on urea, formamide, acetamide, N-methylurea, and related compounds. Can J Biochem. 1980 Dec;58(12):1335–1344. doi: 10.1139/o80-181. [DOI] [PubMed] [Google Scholar]
- Gale G. R. Inhibition of urease by hydroxyurea. Biochem Pharmacol. 1965 May;14(5):693–698. doi: 10.1016/0006-2952(65)90086-9. [DOI] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Hu L. T., Nicholson E. B., Jones B. D., Lynch M. J., Mobley H. L. Morganella morganii urease: purification, characterization, and isolation of gene sequences. J Bacteriol. 1990 Jun;172(6):3073–3080. doi: 10.1128/jb.172.6.3073-3080.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Genetic and biochemical diversity of ureases of Proteus, Providencia, and Morganella species isolated from urinary tract infection. Infect Immun. 1987 Sep;55(9):2198–2203. doi: 10.1128/iai.55.9.2198-2203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
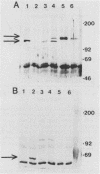
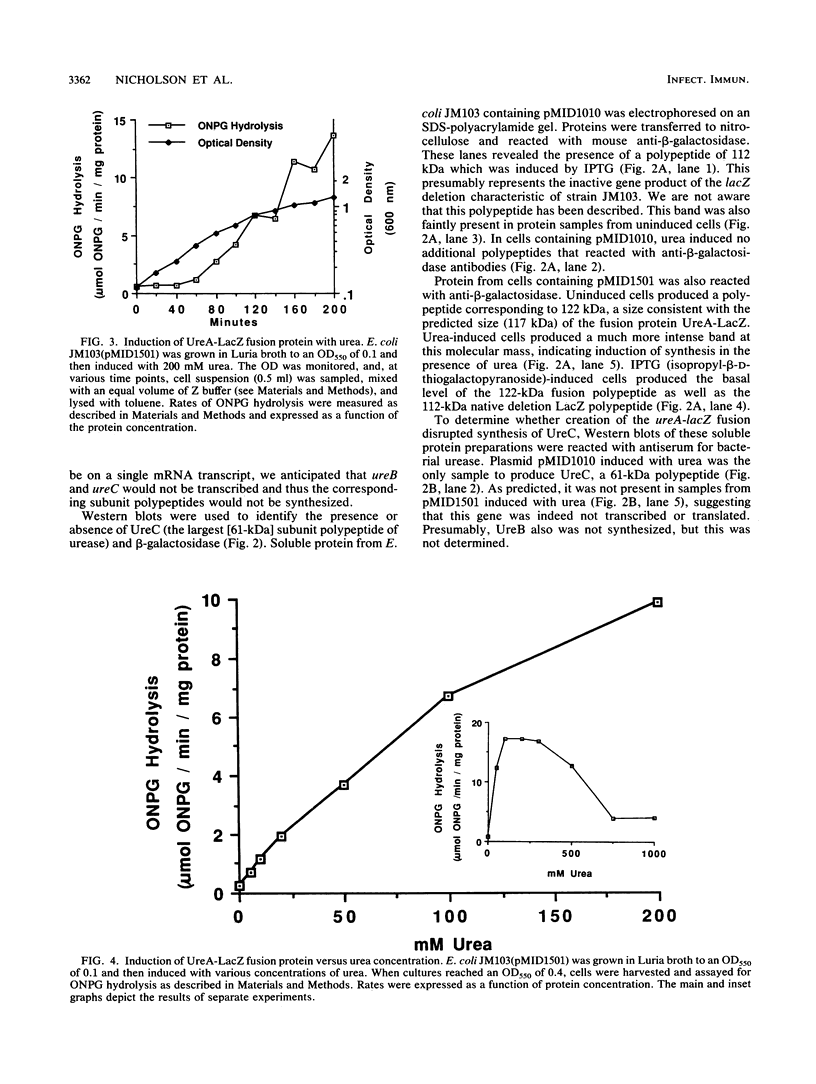
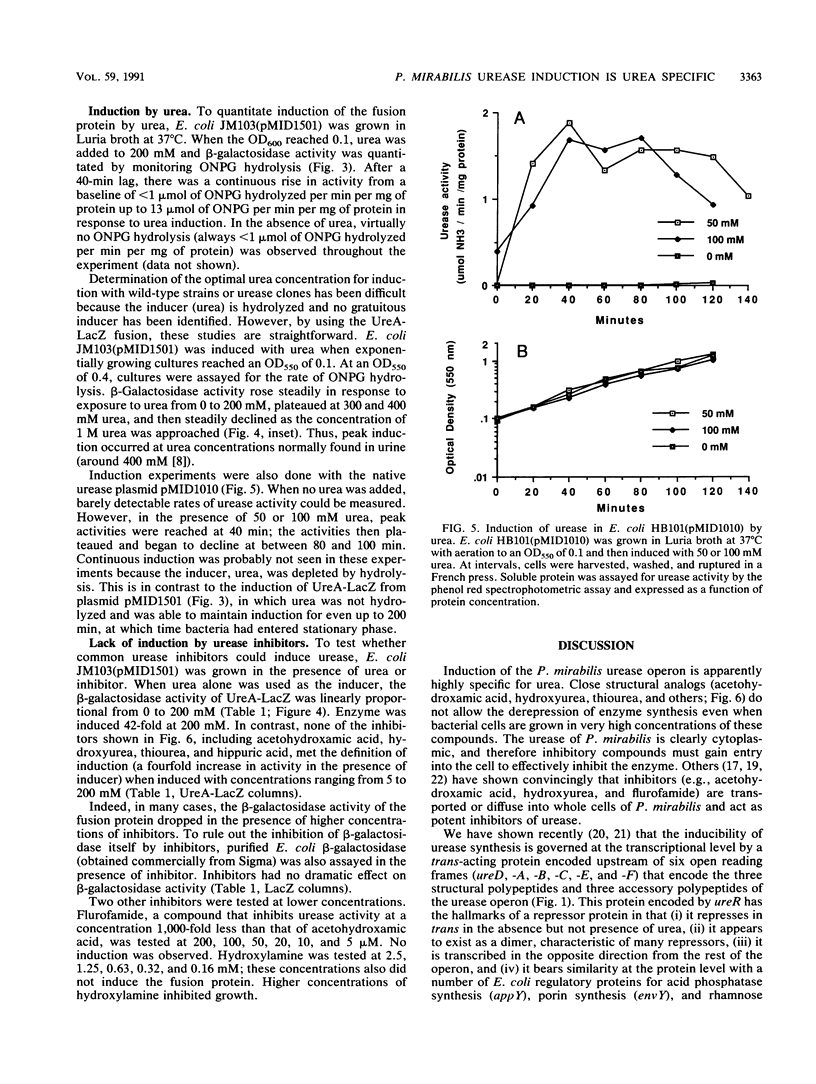
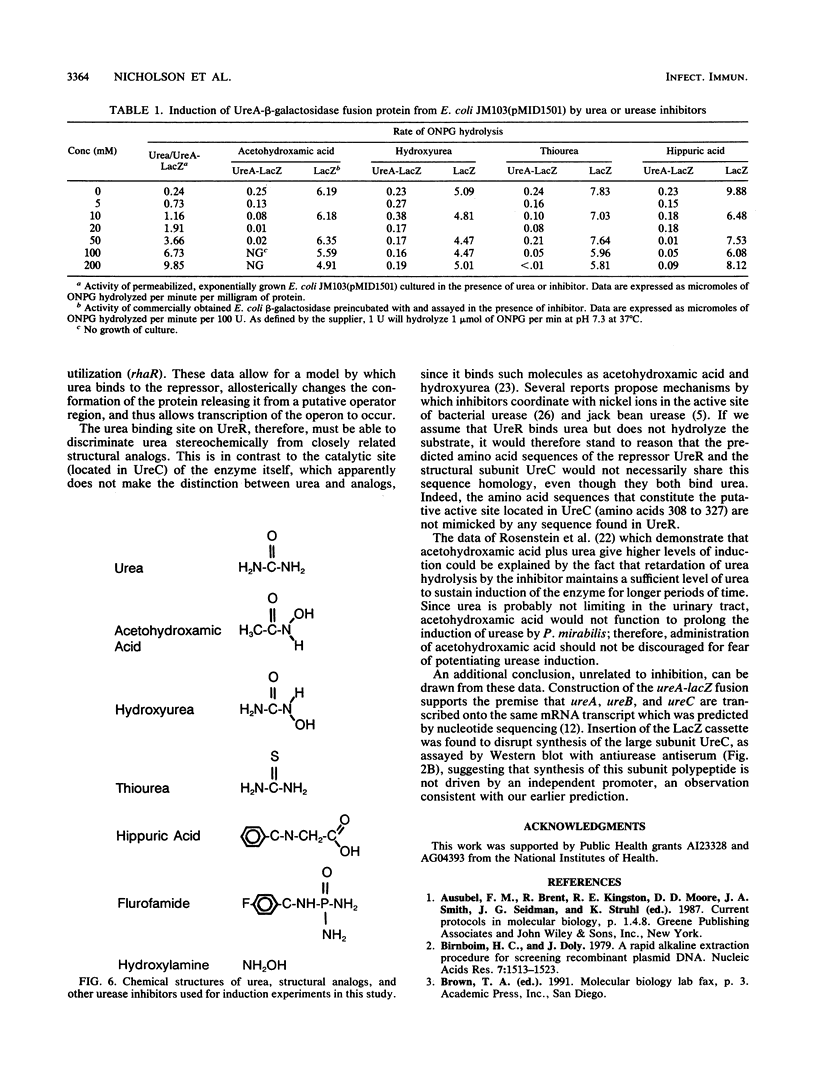
- Jones B. D., Mobley H. L. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol. 1988 Aug;170(8):3342–3349. doi: 10.1128/jb.170.8.3342-3349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: nucleotide sequence determination and comparison with jack bean urease. J Bacteriol. 1989 Dec;171(12):6414–6422. doi: 10.1128/jb.171.12.6414-6422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Millner O. E., Jr, Andersen J. A., Appler M. E., Benjamin C. E., Edwards J. G., Humphrey D. T., Shearer E. M. Flurofamide: a potent inhibitor of bacterial urease with potential clinical utility in the treatment of infection induced urinary stones. J Urol. 1982 Feb;127(2):346–350. doi: 10.1016/s0022-5347(17)53779-9. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Swihart K. G., Welch R. A. Cytotoxicity of the HpmA hemolysin and urease of Proteus mirabilis and Proteus vulgaris against cultured human renal proximal tubular epithelial cells. Infect Immun. 1991 Jun;59(6):2036–2042. doi: 10.1128/iai.59.6.2036-2042.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Hamilton-Miller J. M. Inhibitors of urease as chemotherapeutic agents. Crit Rev Microbiol. 1984;11(1):1–12. doi: 10.3109/10408418409105901. [DOI] [PubMed] [Google Scholar]
- Rosenstein I., Hamilton-Miller J. M., Brumfitt W. The effect of acetohydroxamic acid on the induction of bacterial ureases. Invest Urol. 1980 Sep;18(2):112–114. [PubMed] [Google Scholar]
- Tiedeman A. A., Smith J. M. lacZY gene fusion cassettes with KanR resistance. Nucleic Acids Res. 1988 Apr 25;16(8):3587–3587. doi: 10.1093/nar/16.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M. J., Hausinger R. P. Competitive inhibitors of Klebsiella aerogenes urease. Mechanisms of interaction with the nickel active site. J Biol Chem. 1989 Sep 25;264(27):15835–15842. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]