Abstract
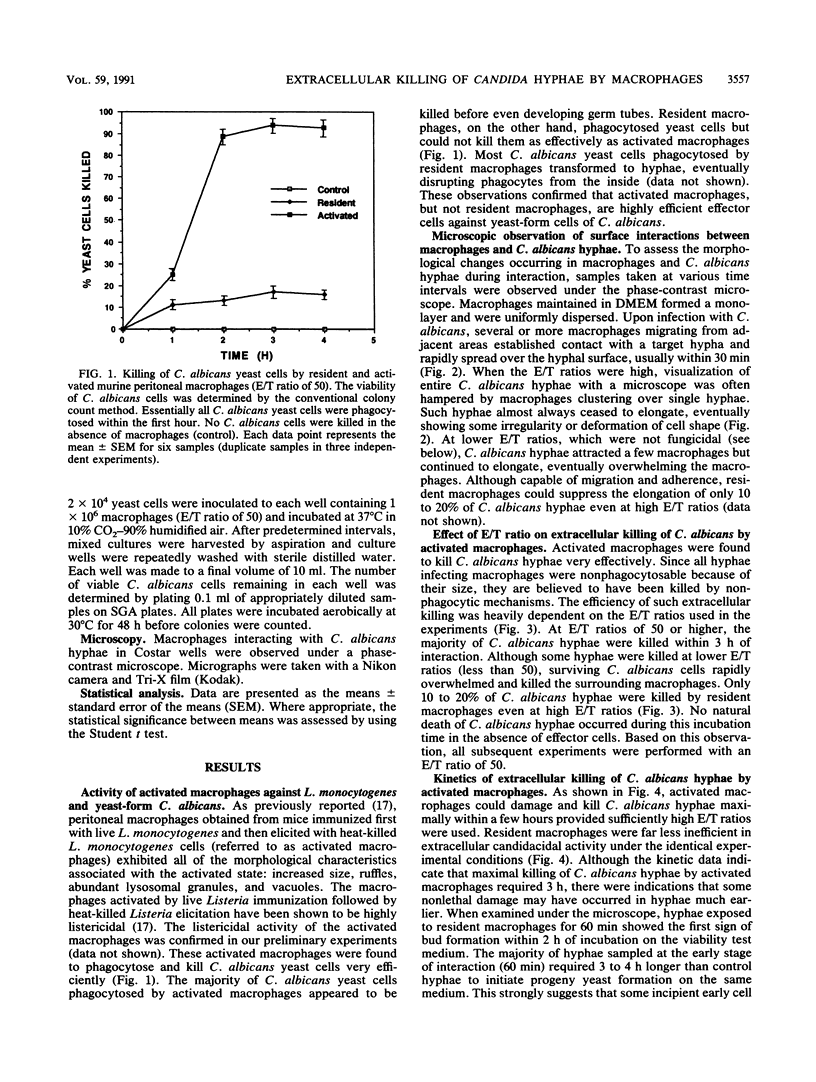
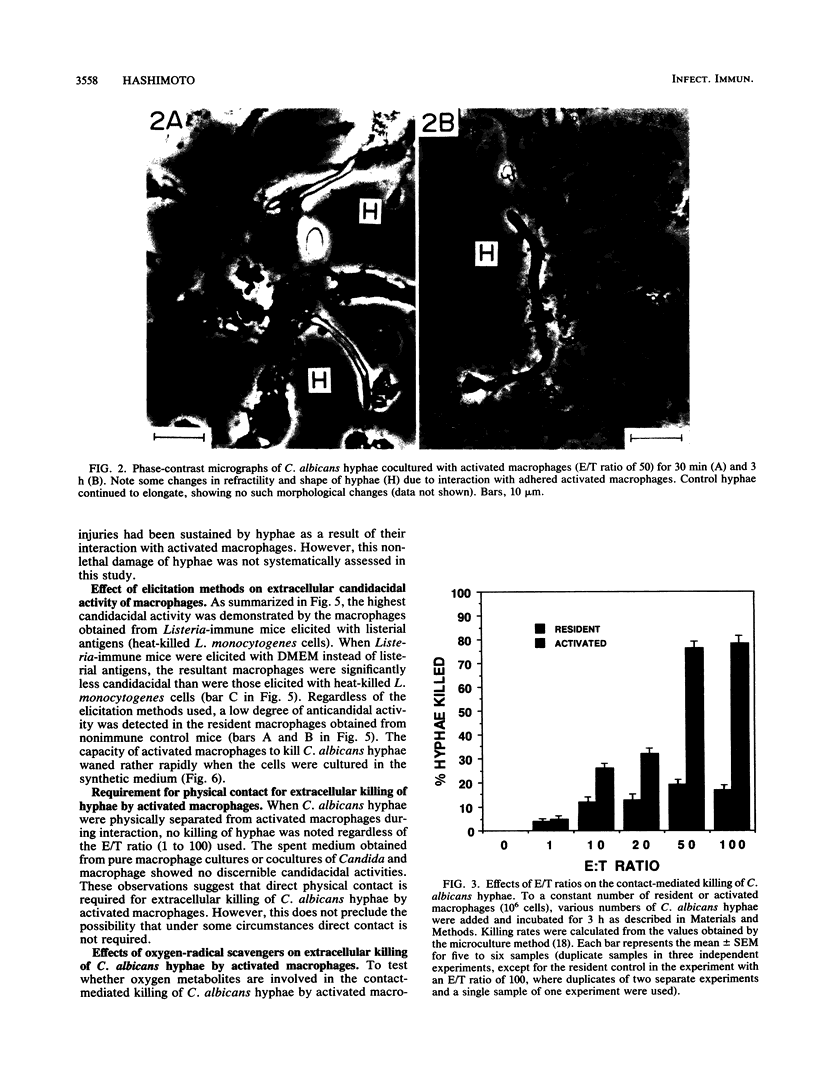
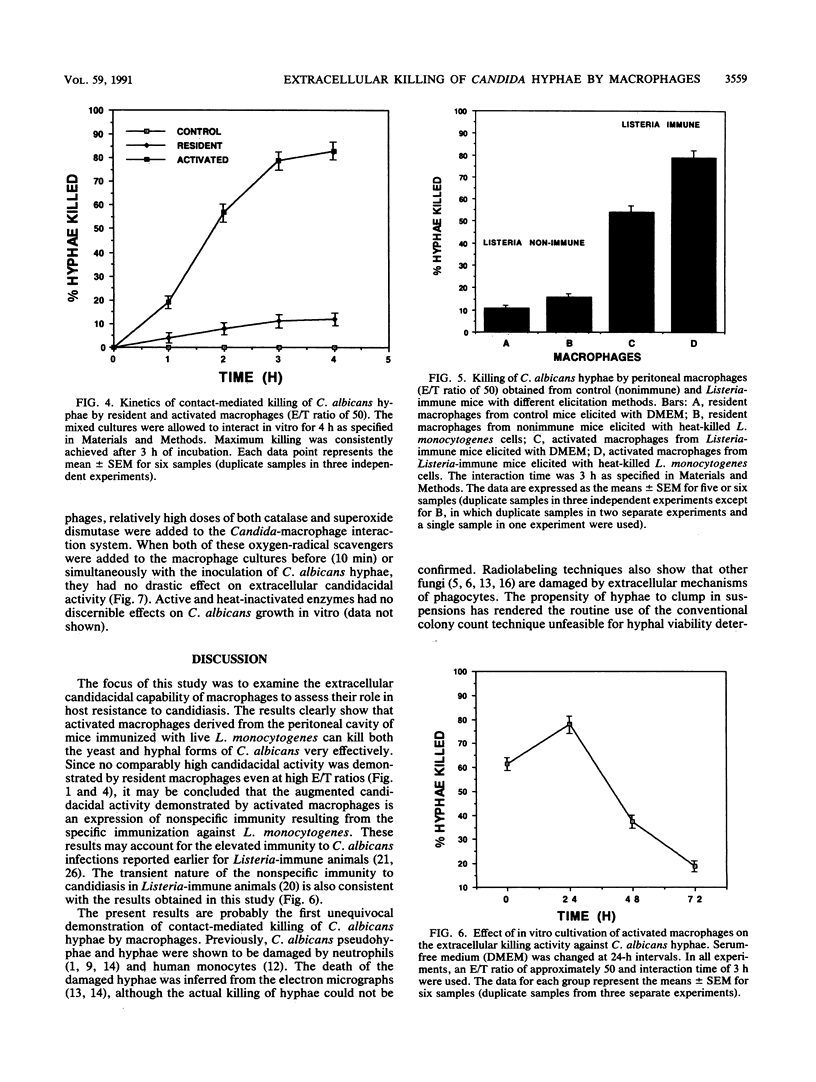
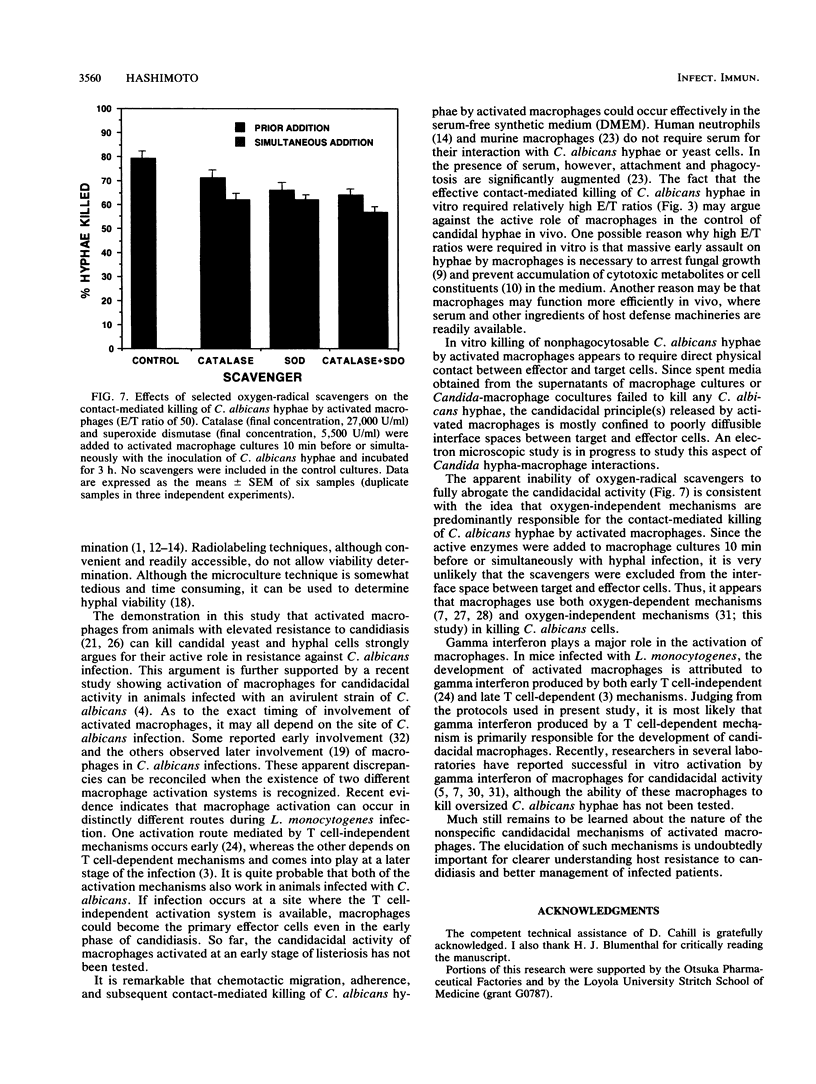
Activated peritoneal macrophages obtained from Listeria-immune mice were demonstrated to kill nonphagocytosable Candida albicans hyphae by contact-mediated mechanisms in a serum-free synthetic medium. The actual killing of hyphae was confirmed by a microculture technique utilizing the dimorphic nature of the fungus. The most efficient candidacidal activity was demonstrated by the macrophages obtained from mice first immunized with live Listeria monocytogenes and then elicited with heat-killed L. monocytogenes cells. Resident macrophages from control mice showed only low candidacidal activity against C. albicans hyphae and yeast cells. Direct physical contact appeared to be required for macrophages to efficiently kill oversized C. albicans hyphae. Efficient in vitro killing of hyphae also required relatively high effector/target cell ratios (50 or higher). The contact-mediated candidacidal activity of activated macrophages was not significantly abrogated by oxygen-radical scavengers, suggesting the involvement of oxygen-independent mechanisms. These results suggest that the enhanced nonspecific immunity to candidiasis seen in Listeria-immune hosts can be attributed, at least in part, to activated fungicidal macrophages. The ability of macrophages to detect and destroy both yeast and hyphal C. albicans cells is clearly an important element of the host defense against candidiasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccarini M., Vecchiarelli A., Cassone A., Bistoni F. Killing of yeast, germ-tube and mycelial forms of Candida albicans by murine effectors as measured by a radiolabel release microassay. J Gen Microbiol. 1985 Mar;131(3):505–513. doi: 10.1099/00221287-131-3-505. [DOI] [PubMed] [Google Scholar]
- Baghian A., Lee K. W. Role of activated macrophages in resistance to systemic candidosis. J Leukoc Biol. 1988 Sep;44(3):166–171. doi: 10.1002/jlb.44.3.166. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bistoni F., Verducci G., Perito S., Vecchiarelli A., Puccetti P., Marconi P., Cassone A. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J Med Vet Mycol. 1988;26(5):285–299. doi: 10.1080/02681218880000401. [DOI] [PubMed] [Google Scholar]
- Brummer E., Morrison C. J., Stevens D. A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985 Sep;49(3):724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Stevens D. A. Candidacidal mechanisms of peritoneal macrophages activated with lymphokines or gamma-interferon. J Med Microbiol. 1989 Mar;28(3):173–181. doi: 10.1099/00222615-28-3-173. [DOI] [PubMed] [Google Scholar]
- Brummer E., Stevens D. A. Fungicidal mechanisms of activated macrophages: evidence for nonoxidative mechanisms for killing of Blastomyces dermatitidis. Infect Immun. 1987 Dec;55(12):3221–3224. doi: 10.1128/iai.55.12.3221-3224.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Haque A., Auriault C., Joseph M. Macrophages as effector cells in helminth infections. Trans R Soc Trop Med Hyg. 1983;77(5):631–635. doi: 10.1016/0035-9203(83)90191-8. [DOI] [PubMed] [Google Scholar]
- Cockayne A., Odds F. C. Interactions of Candida albicans yeast cells, germ tubes and hyphae with human polymorphonuclear leucocytes in vitro. J Gen Microbiol. 1984 Mar;130(3):465–471. doi: 10.1099/00221287-130-3-465. [DOI] [PubMed] [Google Scholar]
- Danley D. L., Polakoff J. Rapid killing of monocytes in vitro by Candida albicans yeast cells. Infect Immun. 1986 Jan;51(1):307–313. doi: 10.1128/iai.51.1.307-313.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. R., Denning T. J. Candida albicans and the fungicidal activity of the blood. Sabouraudia. 1972 Nov;10(3):301–312. [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C. Monocyte-mediated serum-independent damage to hyphal and pseudohyphal forms of Candida albicans in vitro. J Clin Invest. 1981 Jan;67(1):173–182. doi: 10.1172/JCI110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Huber E., Haudenschild C. C. Mechanisms of destruction of Aspergillus fumigatus hyphae mediated by human monocytes. J Infect Dis. 1983 Mar;147(3):474–483. doi: 10.1093/infdis/147.3.474. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J., Schroit A. J. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988 Nov 15;948(2):151–173. doi: 10.1016/0304-419x(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Harrington-Fowler L., Henson P. M., Wilder M. S. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981 Jul;33(1):11–16. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg B. J., van 't Wout J. W., van Furth R. Role of granulocytes in increased host resistance to Candida albicans induced by recombinant interleukin-1. Infect Immun. 1990 Oct;58(10):3319–3324. doi: 10.1128/iai.58.10.3319-3324.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra S., Balish E. Immunity to Candida albicans induced by Listeria monocytogenes. Infect Immun. 1974 Jul;10(1):72–82. doi: 10.1128/iai.10.1.72-82.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C. J., Gordon P., Hashimoto T. Enhanced killing of Candida albicans by cultured peritoneal exudate cells treated with SM-1213, a synthetic immunomodulator. Antimicrob Agents Chemother. 1984 Jul;26(1):74–77. doi: 10.1128/aac.26.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Cutler J. E. In vitro studies of the interaction of murine phagocytic cells with Candida albicans. J Reticuloendothel Soc. 1981 Jan;29(1):23–34. [PubMed] [Google Scholar]
- Mára M., Průchová J., John C., Smerák P., Sír Z. Resistance to Mycobacterium tuberculosis H37Rv infection induced by Listeria and mycobacterial lipids. Folia Microbiol (Praha) 1981;26(1):52–58. doi: 10.1007/BF02927223. [DOI] [PubMed] [Google Scholar]
- Nakane A., Numata A., Asano M., Kohanawa M., Chen Y., Minagawa T. Evidence that endogenous gamma interferon is produced early in Listeria monocytogenes infection. Infect Immun. 1990 Jul;58(7):2386–2388. doi: 10.1128/iai.58.7.2386-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to Candida albicans. Microbiol Rev. 1980 Dec;44(4):660–682. doi: 10.1128/mr.44.4.660-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada M., Kubo A., Nishimura T., Kakita T., Moriguchi T., Yamamoto K., Uchino H. Candidacidal activity of monocyte-derived human macrophages: relationship between Candida killing and oxygen radical generation by human macrophages. J Leukoc Biol. 1987 Apr;41(4):289–294. doi: 10.1002/jlb.41.4.289. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Todisco T., Puliti M., Dottorini M., Bistoni F. Modulation of anti-Candida activity of human alveolar macrophages by interferon-gamma or interleukin-1-alpha. Am J Respir Cell Mol Biol. 1989 Jul;1(1):49–55. doi: 10.1165/ajrcmb/1.1.49. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kagaya K., Yamada T., Fukazawa Y. Mechanism for candidacidal activity in macrophages activated by recombinant gamma interferon. Infect Immun. 1991 Feb;59(2):521–528. doi: 10.1128/iai.59.2.521-528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG G. The process of invasion and the persistence of Candida albicans injected intraperitoneally into mice. J Infect Dis. 1958 Mar-Apr;102(2):114–120. doi: 10.1093/infdis/102.2.114. [DOI] [PubMed] [Google Scholar]
- van 't Wout J. W., Linde I., Leijh P. C., van Furth R. Contribution of granulocytes and monocytes to resistance against experimental disseminated Candida albicans infection. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):736–741. doi: 10.1007/BF01975039. [DOI] [PubMed] [Google Scholar]



