Abstract
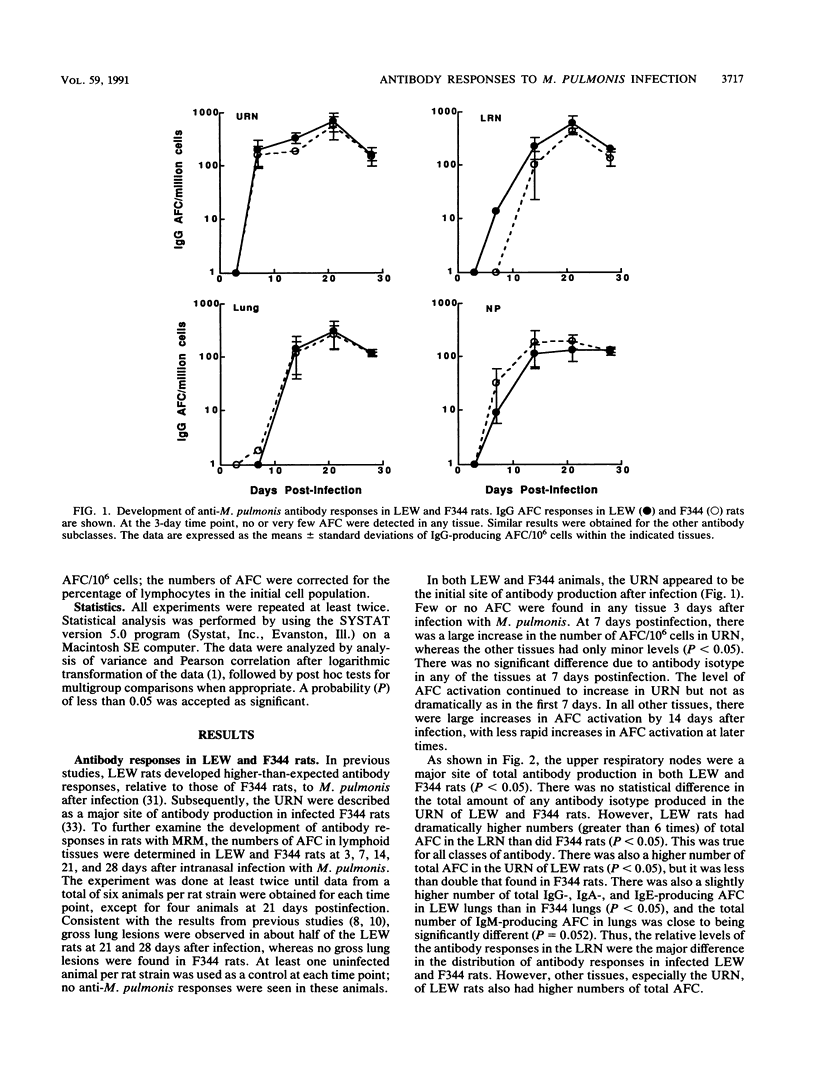
Murine respiratory mycoplasmosis resulting from Mycoplasma pulmonis infection in rats provides a useful model for the study of immunological and inflammatory mechanisms operative in the respiratory tract. We have previously shown that LEW rats develop more severe disease than do F344 rats. To further study the production of antibody responses in chronic respiratory disease due to M. pulmonis infection, we examined the distribution and development of M. pulmonis-specific antibody-forming cells (AFC) in different segments of the respiratory tracts of infected LEW and F344 rats. In these studies, the upper respiratory nodes were the initial site of antibody production after infection and remained the major site for recovery of AFC. Since infected LEW rats had equal or higher numbers of AFC than did infected F344 rats, these results suggest that the level of local antibody production alone is not responsible for the decreased susceptibility of F344 rats to murine respiratory mycoplasmosis. The differences in total antibody responses appear to be due to the greater numbers of cells recovered from the tissues of infected LEW rats compared with those recovered from F344 rats, suggesting that LEW rats may have greater production of chemotactic factors. Also, we demonstrate that nonspecific activation and/or recruitment of B cells occurs in the respiratory tracts of both LEW and F344 rats after infection with M. pulmonis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bice D. E., Degen M. A., Harris D. L., Muggenburg B. A. Recruitment of antibody-forming cells in the lung after local immunization is nonspecific. Am Rev Respir Dis. 1982 Oct;126(4):635–639. doi: 10.1164/arrd.1982.126.4.635. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradley P. A., Bourne F. J., Brown P. J. The respiratory tract immune system in the pig. I. Distribution of immunoglobulin-containing cells in the respiratory tract mucosa. Vet Pathol. 1976;13(2):81–89. doi: 10.1177/030098587601300201. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Murphy B. R., Radl J., Haaijman J. J., Mestecky J. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J Clin Microbiol. 1985 Aug;22(2):259–264. doi: 10.1128/jcm.22.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Davis J. K. Protective effect of vaccination against Mycoplasma pulmonis respiratory disease in rats. Infect Immun. 1978 Jul;21(1):69–75. doi: 10.1128/iai.21.1.69-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Cassell G. H. Murine respiratory mycoplasmosis in LEW and F344 rats: strain differences in lesion severity. Vet Pathol. 1982 May;19(3):280–293. doi: 10.1177/030098588201900306. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Simecka J. W., Williamson J. S., Ross S. E., Juliana M. M., Thorp R. B., Cassell G. H. Nonspecific lymphocyte responses in F344 and LEW rats: susceptibility to murine respiratory mycoplasmosis and examination of cellular basis for strain differences. Infect Immun. 1985 Jul;49(1):152–158. doi: 10.1128/iai.49.1.152-158.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guidice R. A., Barile M. F. Immunofluorescent procedures for mycoplasma identification. Dev Biol Stand. 1974;23:134–137. [PubMed] [Google Scholar]
- Gadaleanu V., Mellbye O. J. Immunofluorescence studies of humoral immune responses in the rat lung induced by respiratory and systemic immunization with ovalbumin. Cell Mol Biol. 1982;28(3):307–312. [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Huang C. H., Collier A. M., Clyde W. A., Jr Demonstration of antibodies to Mycoplasma pneumoniae attachment protein in human sera and respiratory secretions. Infect Immun. 1983 Jul;41(1):437–439. doi: 10.1128/iai.41.1.437-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Mibu Y., Shimokaway Y., Hayashi H. Lymphocyte chemotaxis in inflammation. X. Heterogeneity of chemotactic responsiveness in human T subsets towards lymphocyte chemotactic factors from delayed hypersensitivity reaction site. Immunology. 1985 Jul;55(3):473–479. [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Davidson S., Lindenbaum E. S. Mitogenicity and pathogenicity of Mycoplasma pulmonis in rats. I. Atypical interstitial pneumonia induced by mitogenic myeoplasmal membranes. J Infect Dis. 1981 Jan;143(1):55–62. doi: 10.1093/infdis/143.1.55. [DOI] [PubMed] [Google Scholar]
- Naot Y., Davidson S., Lindenbaum E. S. Role of mitogenicity in pathogenicity of mycoplasmas for murine hosts. Ann Microbiol (Paris) 1984 Jan-Feb;135A(1):95–101. doi: 10.1016/s0769-2609(84)80064-2. [DOI] [PubMed] [Google Scholar]
- Naot Y., Ginsburg H. Activation of B lymphocytes by mycoplasma mitogen(s). Immunology. 1978 Apr;34(4):715–720. [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Lis E., Siman-Tov R., Brunner H. Comparison of enzyme-linked immunosorbent assay and radioimmunoprecipitation test for detection of immunoglobulin A antibodies to Mycoplasma pneumoniae in nasal secretions. J Clin Microbiol. 1986 Nov;24(5):892–893. doi: 10.1128/jcm.24.5.892-893.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. F., Davis J. K., Blalock D. K., Thorp R. B., Simecka J. W., Cassell G. H. Pulmonary clearance of Mycoplasma pulmonis in C57BL/6N and C3H/HeN mice. Infect Immun. 1987 Nov;55(11):2631–2635. doi: 10.1128/iai.55.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A., von Maur R. K., Ishizaka K., Norman P. S., Lichtenstein L. M. IgA and IgG anti-ragweed antibodies in nasal secretions. Quantitative measurements of antibodies and correlation with inhibition of histamine release. J Clin Invest. 1976 Apr;57(4):1041–1050. doi: 10.1172/JCI108346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeb T. R., Davidson M. K., Lindsey J. R. Intracage ammonia promotes growth of Mycoplasma pulmonis in the respiratory tract of rats. Infect Immun. 1982 Oct;38(1):212–217. doi: 10.1128/iai.38.1.212-217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano R., Husband A. J., Clancy R. L. Contribution of intraperitoneal immunization to the local immune response in the respiratory tract of sheep. Immunology. 1984 Oct;53(2):375–384. [PMC free article] [PubMed] [Google Scholar]
- Shvartsman Y. S., Agranovskaya E. N., Zykov M. P. Formation of secretory and circulating antibodies after immunization with live and inactivated influenza virus vaccines. J Infect Dis. 1977 May;135(5):697–705. doi: 10.1093/infdis/135.5.697. [DOI] [PubMed] [Google Scholar]
- Simecka J. W., Cassell G. H. Serum antibody and cellular responses in LEW and F344 rats after immunization with Mycoplasma pulmonis antigens. Infect Immun. 1987 Mar;55(3):731–735. doi: 10.1128/iai.55.3.731-735.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simecka J. W., Davis J. K., Cassell G. H. Serum antibody does not account for differences in the severity of chronic respiratory disease caused by Mycoplasma pulmonis in LEW and F344 rats. Infect Immun. 1989 Nov;57(11):3570–3575. doi: 10.1128/iai.57.11.3570-3575.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simecka J. W., Davis J. K., Cassell G. H. Specific vs. nonspecific immune responses in murine respiratory mycoplasmosis. Isr J Med Sci. 1987 May;23(5):485–489. [PubMed] [Google Scholar]
- Simecka J. W., Patel P., Davis J. K., Cassell G. H. Upper respiratory tract is the major site of antibody production in mycoplasmal induced chronic respiratory disease. Reg Immunol. 1989 Nov-Dec;2(6):385–389. [PubMed] [Google Scholar]
- Spinella S., Milon G., Hontebeyrie-Joskowicz M. A CD4+ TH2 cell line isolated from mice chronically infected with Trypanosoma cruzi induces IgG2 polyclonal response in vivo. Eur J Immunol. 1990 May;20(5):1045–1051. doi: 10.1002/eji.1830200515. [DOI] [PubMed] [Google Scholar]
- Taylor G., Howard C. J. Class-specific antibody responses to Mycoplasma pulmonis in sera and lungs of infected and vaccinated mice. Infect Immun. 1980 Sep;29(3):1160–1168. doi: 10.1128/iai.29.3.1160-1168.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
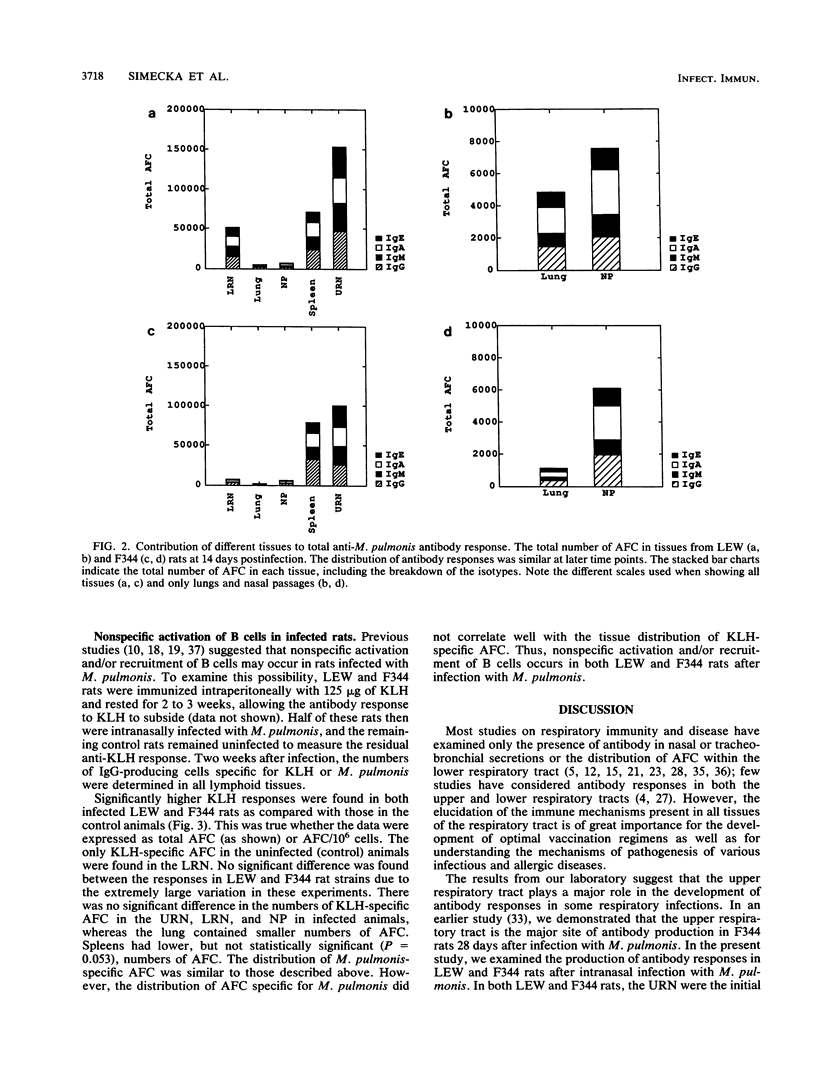
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]
- Williamson J. S., Davis J. K., Cassell G. H. Polyclonal activation of rat splenic lymphocytes after in vivo administration of Mycoplasma pulmonis and its relation to in vitro response. Infect Immun. 1986 May;52(2):594–599. doi: 10.1128/iai.52.2.594-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


