Abstract
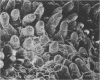
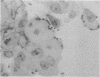
The GV strain (serotype O103:H2:K-), originally isolated from a diarrheic rabbit, is an enteropathogenic Escherichia coli strain that produces diarrhea without synthesizing the classical enterotoxins and that is not invasive. This strain is characterized by a 117-kb plasmid (pREC-1). Histological study of the gut by scanning electron microscopy and transmission electron microscopy was performed on the GV strain, on a derivative strain cured of pREC-1, and on transconjugants obtained by transfer of pREC-1 to nonpathogenic strains E. coli K-12 and 6100, not belonging to the O103 serogroup. The GV strain adhered to the epithelial cells of the ileum and large intestine, whereas the cured GV strain did not. Transfer of plasmid pREC-1 to E. coli K-12 or 6100 allowed the bacteria to attach to the intestinal mucosa in the same manner as that of the wild-type GV strain. Thus, pREC-1 seems to play an important role in attachment to and colonization of the intestinal tract of rabbits by E. coli serogroup O103. Scanning electron microscopy showed numerous bacteria attached together and closely associated with intestinal villi. Transmission electron microscopy revealed effacing lesions characteristic of enteropathogenic E. coli strains: effacing of microvilli and cuplike projections (pedestal formations) associated with an acute inflammatory and hemorrhagic response. In contrast with the results reported for rabbit pathogenic O15 strains, it appeared that the Peyer's patches were not involved in the early stages of infection with the O103 GV strain. This strain may represent a model for the study of the virulence and pathogenic effects of enteropathogenic and enterohemorrhagic E. coli strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Camguilhem R., Lebas F., Labie C. Reproduction expérimentale chez le lapin en engraissement d'une diarrhée provoquée par une souche de Escherichia coli de sérogroupe O-103. Ann Rech Vet. 1986;17(4):409–424. [PubMed] [Google Scholar]
- Camguilhem R., Milon A. Biotypes and O serogroups of Escherichia coli involved in intestinal infections of weaned rabbits: clues to diagnosis of pathogenic strains. J Clin Microbiol. 1989 Apr;27(4):743–747. doi: 10.1128/jcm.27.4.743-747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Inman L. R., Blake R. K. Production of diarrhea in the rabbit by a mutant of Escherichia coli (RDEC-1) that does not express adherence (AF/R1) pili. J Infect Dis. 1989 Jul;160(1):136–141. doi: 10.1093/infdis/160.1.136. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Formal S. B., Schad P. A., Boedeker E. C. Genetic transfer of a mucosal adherence factor (R1) from an enteropathogenic Escherichia coli strain into a Shigella flexneri strain and the phenotypic suppression of this adherence factor. J Infect Dis. 1983 Apr;147(4):711–723. doi: 10.1093/infdis/147.4.711. [DOI] [PubMed] [Google Scholar]
- Hall G. A., Dorn C. R., Chanter N., Scotland S. M., Smith H. R., Rowe B. Attaching and effacing lesions in vivo and adhesion to tissue culture cells of Vero-cytotoxin-producing Escherichia coli belonging to serogroups O5 and O103. J Gen Microbiol. 1990 Apr;136(4):779–786. doi: 10.1099/00221287-136-4-779. [DOI] [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R. Peyer's patch lymphoid follicle epithelial adherence of a rabbit enteropathogenic Escherichia coli (strain RDEC-1). Role of plasmid-mediated pili in initial adherence. J Clin Invest. 1984 Jul;74(1):90–95. doi: 10.1172/JCI111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R. Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer's patch in Escherichia coli diarrhea in the rabbit. J Clin Invest. 1983 Jan;71(1):1–8. doi: 10.1172/JCI110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Milon A., Esslinger J., Camguilhem R. Adhesion of Escherichia coli strains isolated from diarrheic weaned rabbits to intestinal villi and HeLa cells. Infect Immun. 1990 Aug;58(8):2690–2695. doi: 10.1128/iai.58.8.2690-2695.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D., Thompson M. R., Formal S. B. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982 Dec;146(6):763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- O'Brien A. O., Lively T. A., Chen M. E., Rothman S. W., Formal S. B. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet. 1983 Mar 26;1(8326 Pt 1):702–702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- Peeters J. E., Charlier G. J., Halen P. H. Pathogenicity of attaching effacing enteropathogenic Escherichia coli isolated from diarrheic suckling and weanling rabbits for newborn rabbits. Infect Immun. 1984 Dec;46(3):690–696. doi: 10.1128/iai.46.3.690-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters J. E., Charlier G. J., Raeymaekers R. Scanning and transmission electron microscopy of attaching effacing Escherichia coli in weanling rabbits. Vet Pathol. 1985 Jan;22(1):54–59. doi: 10.1177/030098588502200109. [DOI] [PubMed] [Google Scholar]
- Peeters J. E., Pohl P., Charlier G. Infectious agents associated with diarrhoea in commercial rabbits: a field study. Ann Rech Vet. 1984;15(3):335–340. [PubMed] [Google Scholar]
- Peeters J. E., Pohl P., Okerman L., Devriese L. A. Pathogenic properties of Escherichia coli strains isolated from diarrheic commercial rabbits. J Clin Microbiol. 1984 Jul;20(1):34–39. doi: 10.1128/jcm.20.1.34-39.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud A., Federighi M., Licois D., Guillot J. F., Joly B. R plasmid in Escherichia coli O103 coding for colonization of the rabbit intestinal tract. Infect Immun. 1991 Jun;59(6):1888–1892. doi: 10.1128/iai.59.6.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Takeuchi A., Inman L. R., O'Hanley P. D., Cantey J. R., Lushbaugh W. B. Scanning and transmission electron microscopic study of Escherichia coli O15 (RDEC-1) enteric infection in rabbits. Infect Immun. 1978 Feb;19(2):686–694. doi: 10.1128/iai.19.2.686-694.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Gibson R., Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989 Apr;57(4):1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Andrews G. P., Fritz D. L., Sjogren R. W., Jr, Boedeker E. C. Characterization of the plasmid from Escherichia coli RDEC-1 that mediates expression of adhesin AF/R1 and evidence that AF/R1 pili promote but are not essential for enteropathogenic disease. Infect Immun. 1988 Aug;56(8):1846–1857. doi: 10.1128/iai.56.8.1846-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]