Abstract
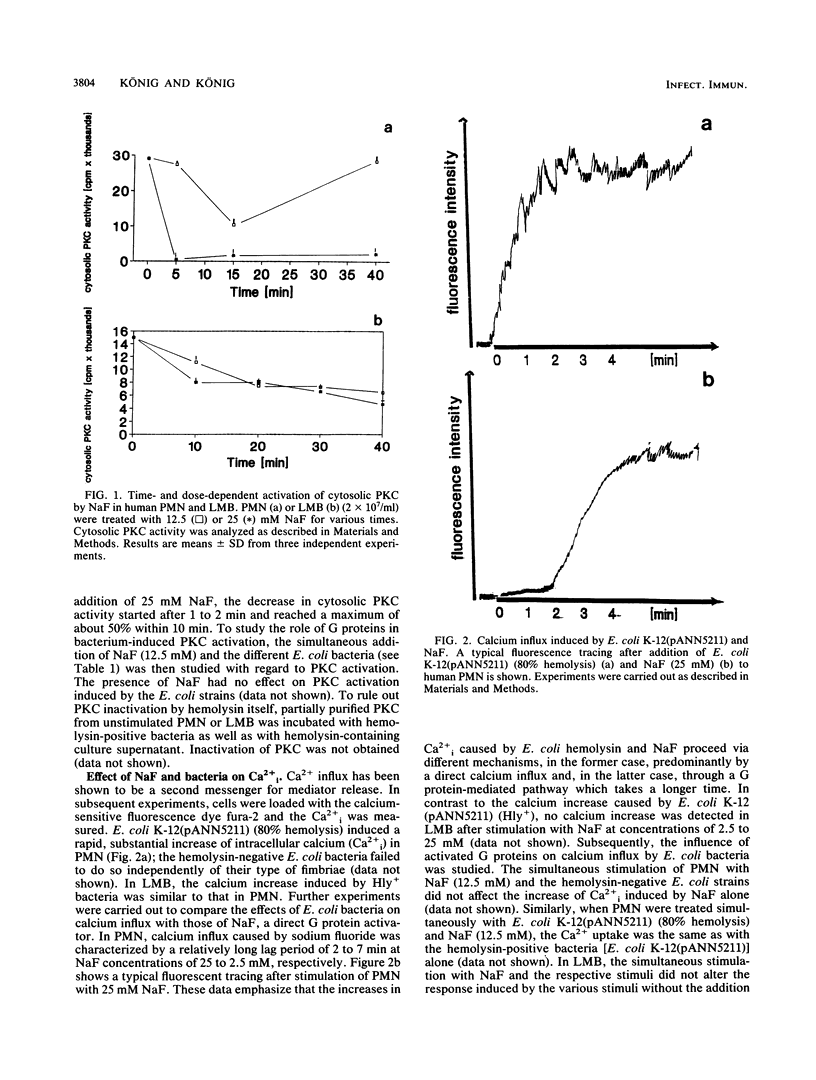
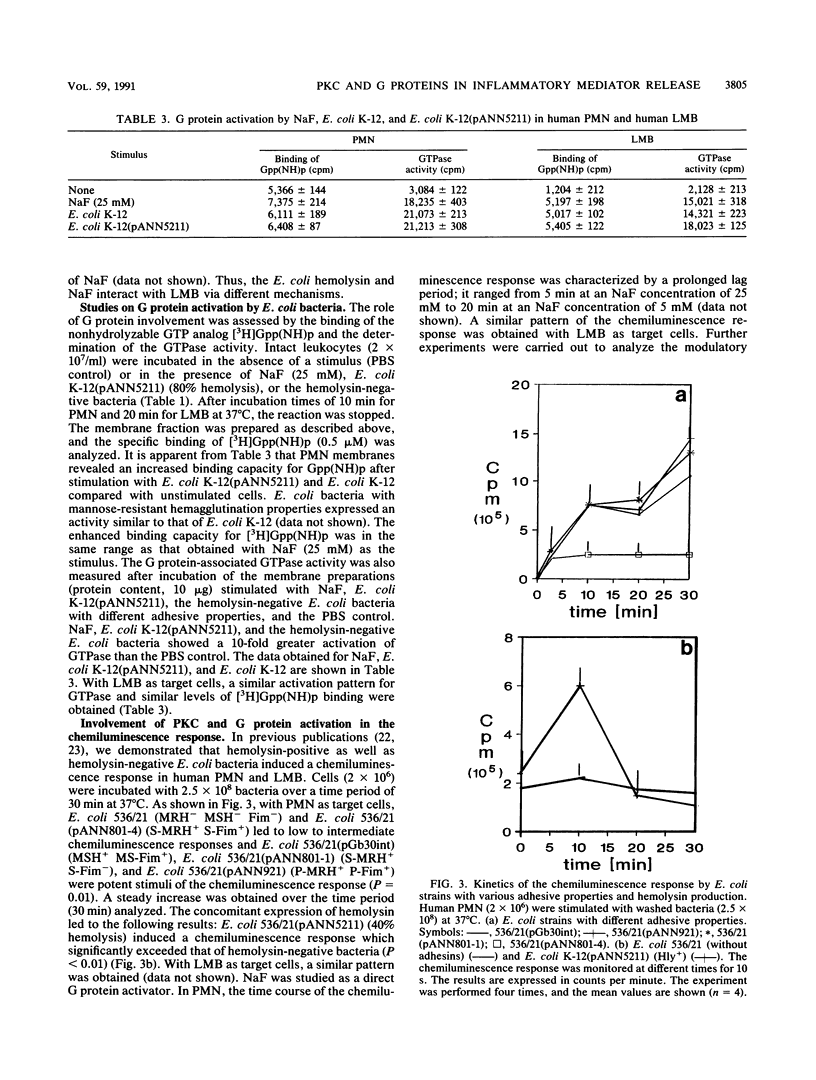
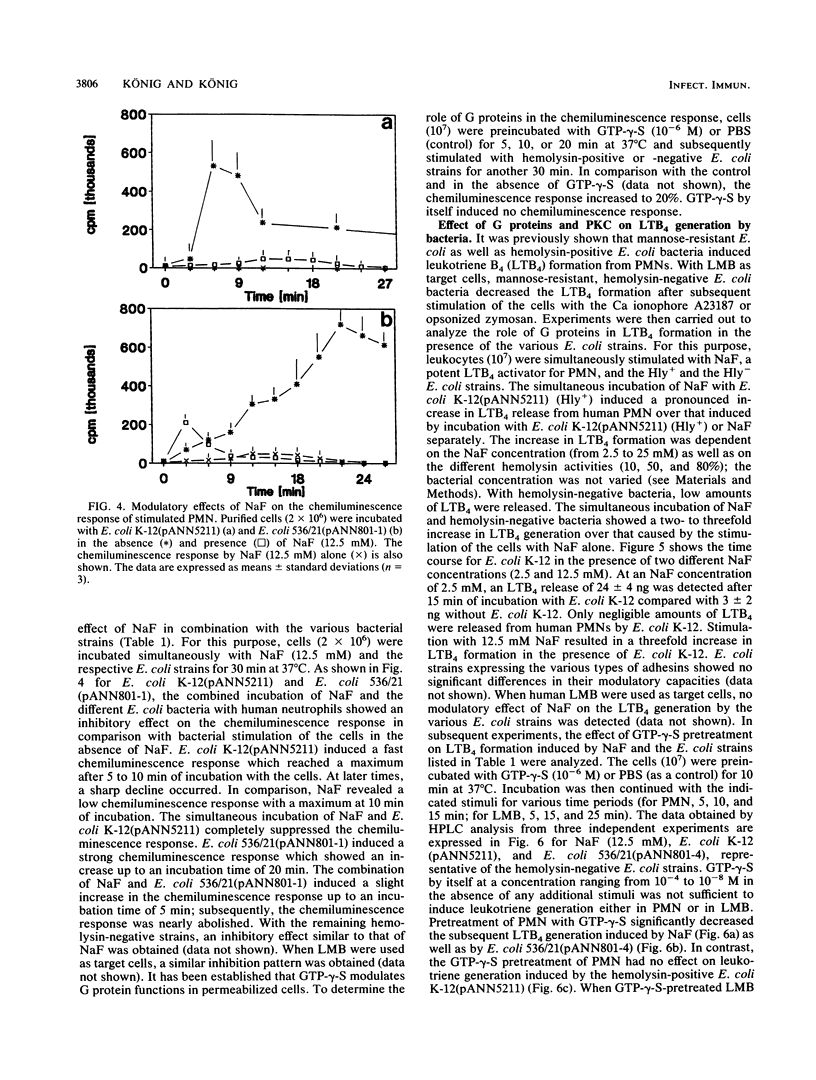
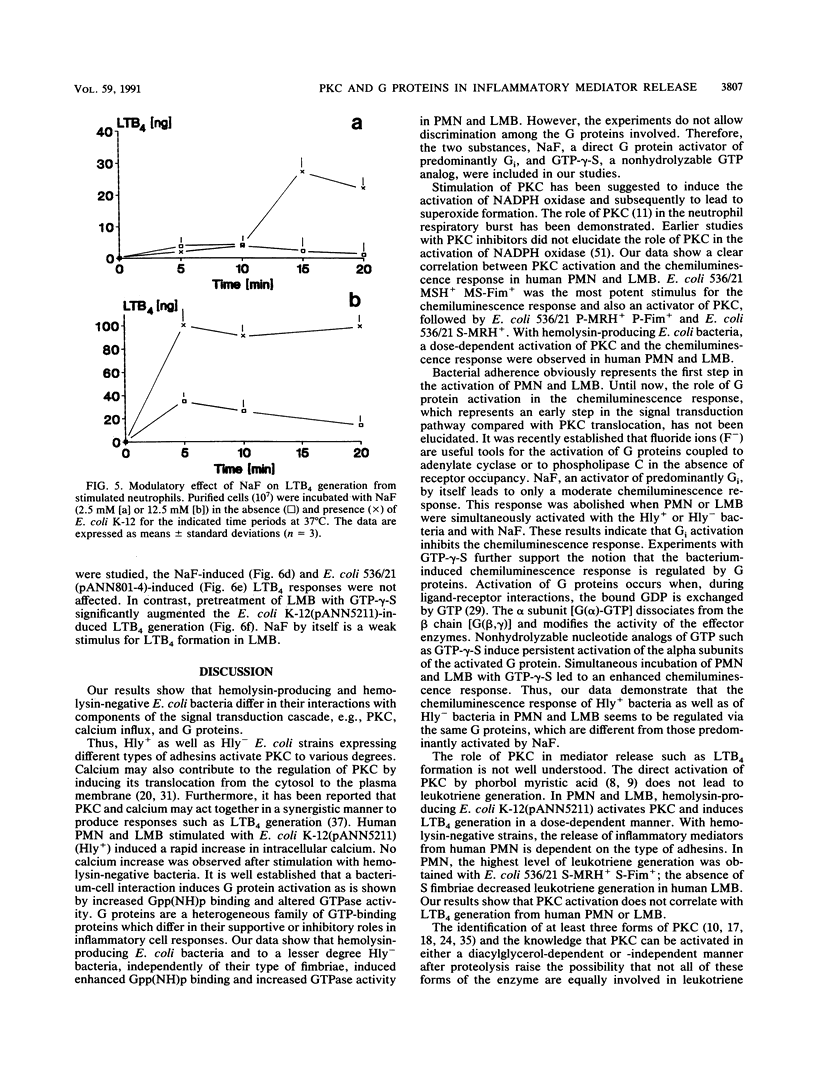
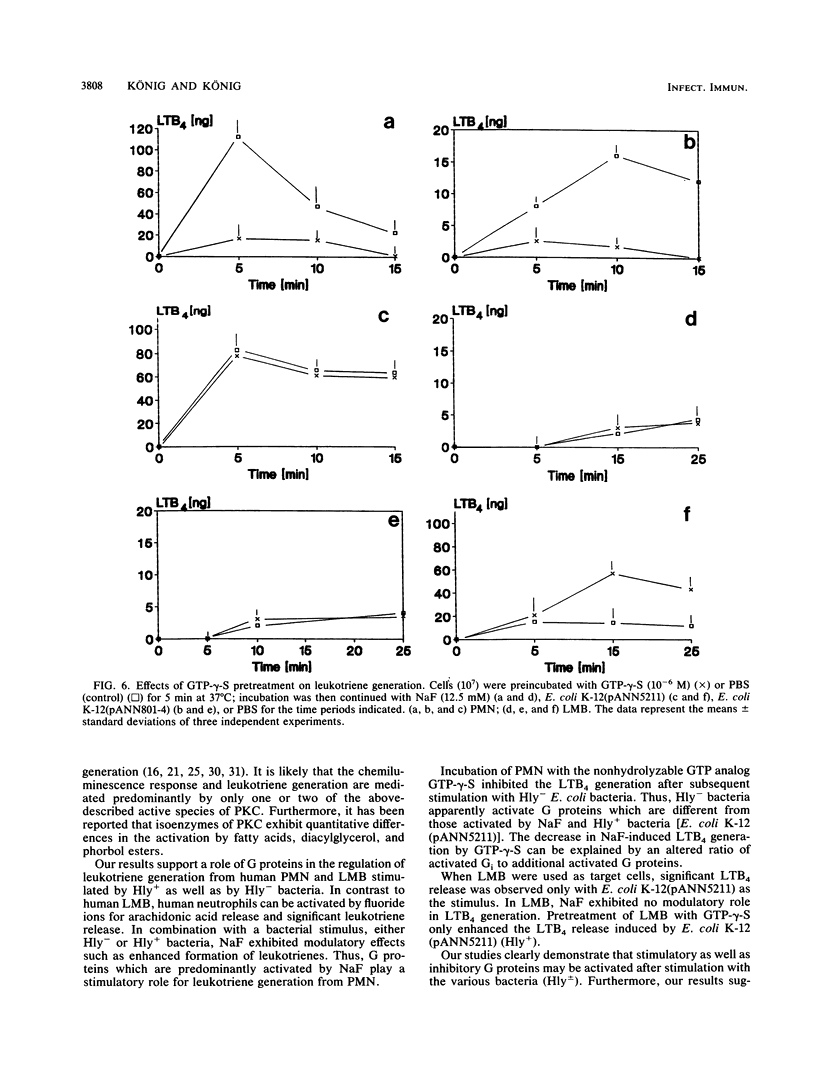
The signal transduction pathway (protein kinase C [PKC], calcium influx, and G protein involvement) was studied with isogenic Escherichia coli strains expressing different types of adhesins (MSH+/- MS-Fim+/-, P-MRH+/- P-Fim+/-, and S-MRH+/- S-Fim+/-) or varying only in the expression of E. coli alpha-hemolysin. As target cells, human polymorphonuclear granulocytes (PMN) and a lymphocyte-monocyte-basophil (LMB) cell suspension were used. The alpha-hemolysin-producing (Hly+) strain E. coli K-12(pANN5211) induced calcium influx in a dose-dependent manner in both cell types. No calcium influx was detected after stimulation with the hemolysin-negative (Hly-) E. coli bacteria independent of the type of fimbriae. With Hly+ bacteria, a dose-dependent activation of PKC was observed in both cell types. The Hly- E. coli K-12 induced PKC to a lesser degree, expressing kinetics different from those of E. coli K-12(pANN5211) (Hly+). E. coli MSH+ MS-Fim+ was the most potent activator for PKC. Membrane preparations from leukocytes stimulated with Hly+ E. coli K-12(pANN5211) showed increased binding of [3H]guanylylimidodiphosphate, a nonhydrolyzable GTP analog, and increased GTPase activity compared with leukocytes stimulated with Hly- E. coli K-12. The amounts of GTPase activation and [3H]guanylylimidodiphosphate binding were similar for all Hly- E. coli bacteria in human PMN as well as in human LMB; no activation was obtained for E. coli bacteria without any type of fimbriae. GTP-gamma-S, a nonhydrolyzable GTP analog, inhibited the leukotriene B4 (LTB4) generation from human PMN by Hly- bacteria, unlike E. coli K-12(pANN5211). However, in the presence of NaF, a predominant activator of Gi, LTB4 generation by Hly+ and by Hly- bacteria was significantly enhanced. For LMBs only LTB4 generation by Hly+ bacteria was increased in the presence of GTP-gamma-S. NaF decreased the chemiluminescence induced by all E. coli strains. Our results thus indicate that (i) Hly+ and Hly- bacteria induce the activation of distinct G proteins, e.g., Gi, to different degrees, (ii) LTB4 generation and chemiluminescence response are differently regulated, and (iii) in comparison with PMN, a different signal transduction pathway is activated by E. coli bacteria in LMBs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Parkos C. A. Identification of novel GTP-binding proteins in the human neutrophil. FEBS Lett. 1988 Jan 18;227(1):66–70. doi: 10.1016/0014-5793(88)81415-7. [DOI] [PubMed] [Google Scholar]
- Boucek M. M., Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science. 1976 Sep 3;193(4256):905–907. doi: 10.1126/science.948752. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brom C., Köller M., Brom J., König W. Effect of sodium fluoride on the generation of lipoxygenase products from human polymorphonuclear granulocytes, mononuclear cells and platelets--indication for the involvement of G proteins. Immunology. 1989 Oct;68(2):240–246. [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen N. O., Larsen C. S., Juhl H., Esmann V. Membrane-associated protein kinases in phorbol ester-activated human polymorphonuclear leukocytes. Biochim Biophys Acta. 1986 Oct 29;884(1):54–59. doi: 10.1016/0304-4165(86)90226-6. [DOI] [PubMed] [Google Scholar]
- Cochet C., Keramidas M., Souvignet C., Chambaz E. M. Phorbol ester-induced alteration of protein kinase C catalytic properties occurs at the membrane level and is not reproduced by physiological stimuli. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1283–1290. doi: 10.1016/s0006-291x(86)80422-3. [DOI] [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Jeng A. Y., Sharkey N. A., Blumberg P. M., Tauber A. I. Activation of the human neutrophil nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase by protein kinase C. J Clin Invest. 1985 Nov;76(5):1932–1938. doi: 10.1172/JCI112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. A reversible process. J Clin Invest. 1979 Apr;63(4):637–647. doi: 10.1172/JCI109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein regulation of receptor signalling. Immunol Today. 1988 Oct;9(10):315–320. doi: 10.1016/0167-5699(88)91325-4. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Appelbaum B. D., Vogler W. R., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase and its substrates in human neutrophils. Biochem Biophys Res Commun. 1983 Mar 29;111(3):847–853. doi: 10.1016/0006-291x(83)91376-1. [DOI] [PubMed] [Google Scholar]
- Housey G. M., O'Brian C. A., Johnson M. D., Kirschmeier P., Weinstein I. B. Isolation of cDNA clones encoding protein kinase C: evidence for a protein kinase C-related gene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1065–1069. doi: 10.1073/pnas.84.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Nakabayashi H., Knopf J. L., Young W. S., 3rd, Huang K. P. Immunochemical identification of protein kinase C isozymes as products of discrete genes. Biochem Biophys Res Commun. 1987 Dec 31;149(3):946–952. doi: 10.1016/0006-291x(87)90500-6. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Kosaka Y., Ogita K., Ase K., Nomura H., Kikkawa U., Nishizuka Y. The heterogeneity of protein kinase C in various rat tissues. Biochem Biophys Res Commun. 1988 Mar 30;151(3):973–981. doi: 10.1016/s0006-291x(88)80461-3. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- König B., König W., Scheffer J., Hacker J., Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986 Dec;54(3):886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König W., König B., Scheffer J., Hacker J., Goebel W. Role of cloned virulence factors (mannose-resistant haemagglutination, mannose-resistant adhesions) from uropathogenic Escherichia coli strains in the release of inflammatory mediators from neutrophils and mast cells. Immunology. 1989 Jul;67(3):401–407. [PMC free article] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Liles W. C., Meier K. E., Henderson W. R. Phorbol myristate acetate and the calcium ionophore A23187 synergistically induce release of LTB4 by human neutrophils: involvement of protein kinase C activation in regulation of the 5-lipoxygenase pathway. J Immunol. 1987 May 15;138(10):3396–3402. [PubMed] [Google Scholar]
- Marre R., Hacker J., Henkel W., Goebel W. Contribution of cloned virulence factors from uropathogenic Escherichia coli strains to nephropathogenicity in an experimental rat pyelonephritis model. Infect Immun. 1986 Dec;54(3):761–767. doi: 10.1128/iai.54.3.761-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Molski T. F., Kanaho Y., Becker E. L., Sha'afi R. I. G-protein dissociation, GTP-GDP exchange and GTPase activity in control and PMA treated neutrophils stimulated by fMet-Leu-Phe. Biochem Biophys Res Commun. 1987 Mar 13;143(2):489–498. doi: 10.1016/0006-291x(87)91380-5. [DOI] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Horecker B. L. The involvement of calpain in the activation of protein kinase C in neutrophils stimulated by phorbol myristic acid. J Biol Chem. 1986 Mar 25;261(9):4101–4105. [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Salamino F., Horecker B. L. Binding of protein kinase C to neutrophil membranes in the presence of Ca2+ and its activation by a Ca2+-requiring proteinase. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6435–6439. doi: 10.1073/pnas.82.19.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S., Nagata K., Ueeda K., Nozawa Y. Stimulation of arachidonic acid release by guanine nucleotide in saponin-permeabilized neutrophils: evidence for involvement of GTP-binding protein in phospholipase A2 activation. Arch Biochem Biophys. 1988 Mar;261(2):375–383. doi: 10.1016/0003-9861(88)90353-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983 Sep 25;258(18):11369–11376. [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Raulf M., König W. Modulation of leukotriene release from human polymorphonuclear leucocytes by PMA and arachidonic acid. Immunology. 1988 May;64(1):51–59. [PMC free article] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad C. F., Parente J. E., Wong K. Use of fluoride ion as a probe for the guanine nucleotide-binding protein involved in the phosphoinositide-dependent neutrophil transduction pathway. FEBS Lett. 1986 Sep 29;206(1):20–24. doi: 10.1016/0014-5793(86)81332-1. [DOI] [PubMed] [Google Scholar]
- Strnad C. F., Wong K. Calcium mobilization in fluoride activated human neutrophils. Biochem Biophys Res Commun. 1985 Nov 27;133(1):161–167. doi: 10.1016/0006-291x(85)91855-8. [DOI] [PubMed] [Google Scholar]
- Tauber A. I. Protein kinase C and the activation of the human neutrophil NADPH-oxidase. Blood. 1987 Mar;69(3):711–720. [PubMed] [Google Scholar]
- Toper R., Aviram A., Aviram I. Fluoride-mediated activation of guinea pig neutrophils. Biochim Biophys Acta. 1987 Dec 10;931(3):262–266. doi: 10.1016/0167-4889(87)90215-1. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E., Olcott M. C., Bell R. M., Merrill A. H., Jr, Lambeth J. D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986 Sep 25;261(27):12616–12623. [PubMed] [Google Scholar]


