Abstract
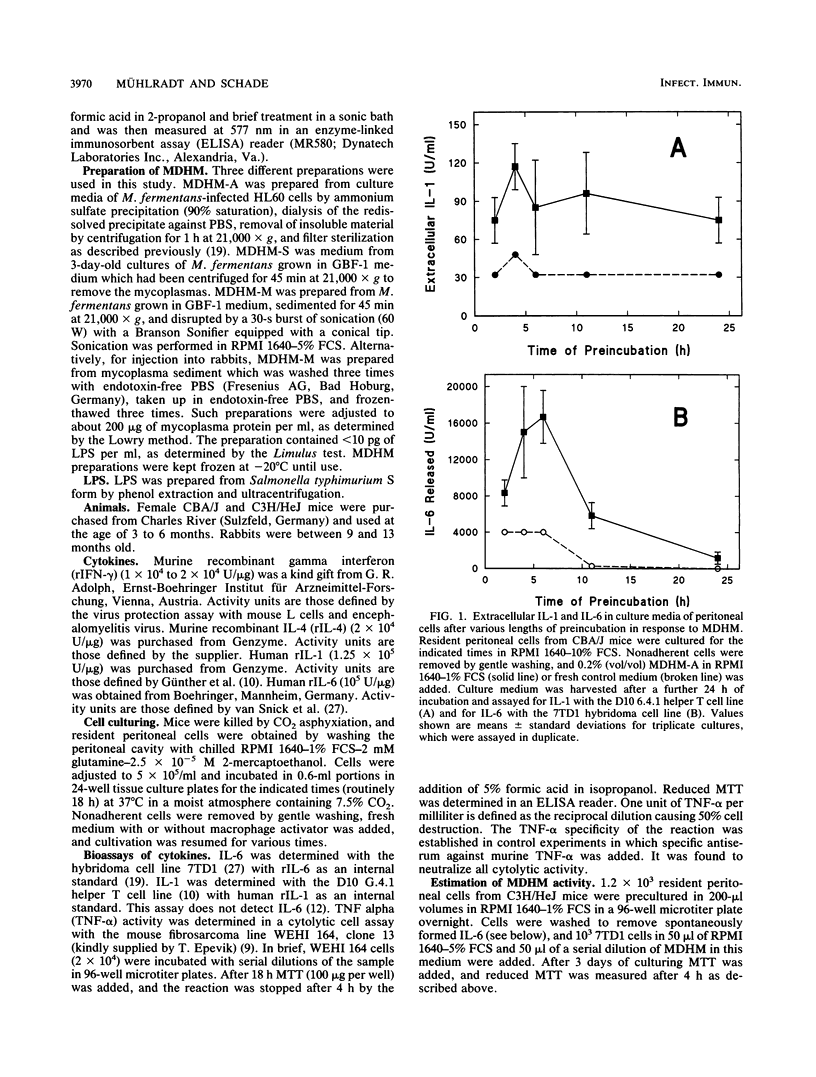
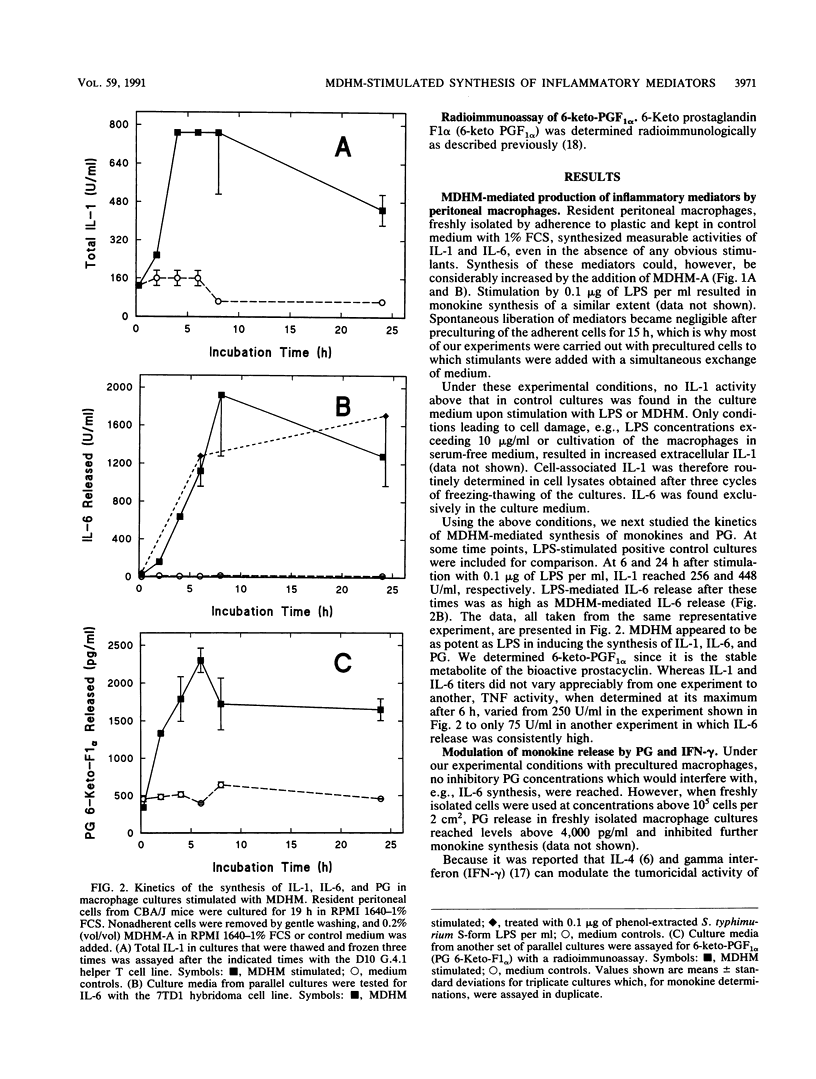
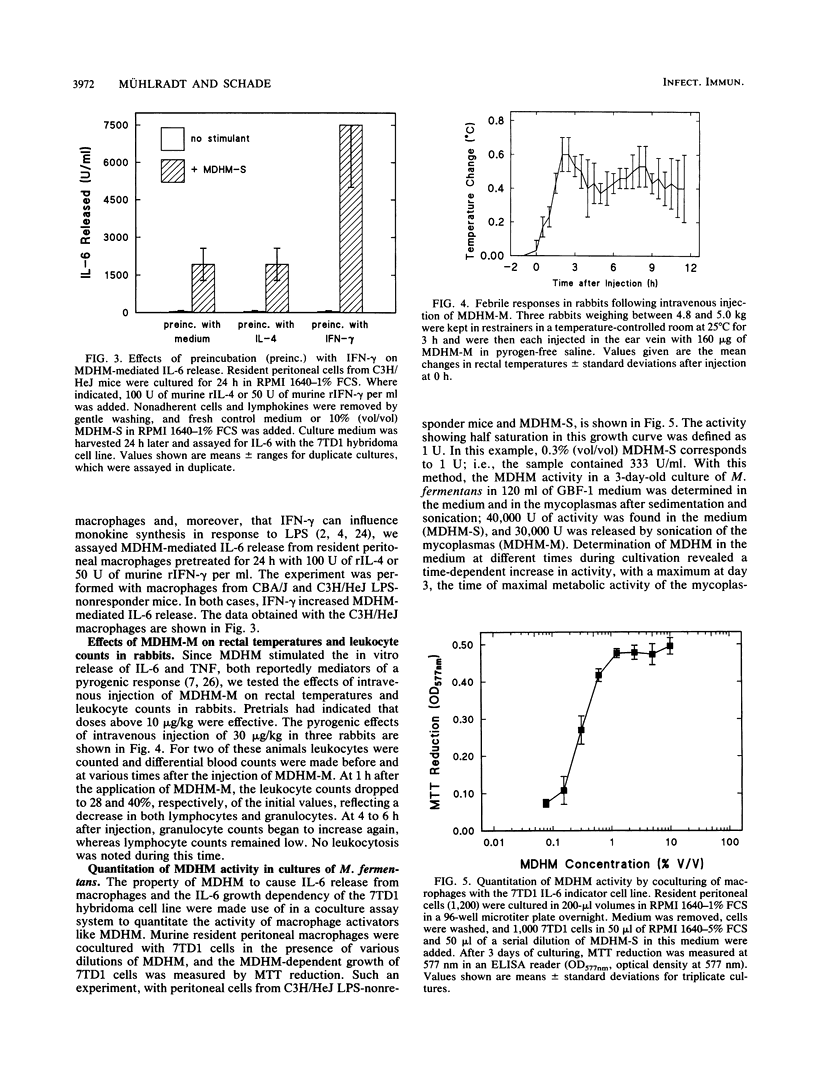
Mycoplasma fermentans-derived high-molecular-weight material (MDHM) was originally discovered because of its capacity to generate, through the induction of monokine synthesis, cytolytic T lymphocytes in concanavalin A-stimulated thymocyte cultures. This study shows that MDHM-activated macrophages not only released interleukin-6 (IL-6) but also exhibited increased synthesis of cell-associated IL-1 as well as liberation of tumor necrosis factor and prostaglandin. We determined 6-keto prostaglandin F1 alpha since it is the stable metabolite of the bioactive prostacyclin. MDHM appeared to be as potent as lipopolysaccharide in inducing the synthesis of these mediators. Priming with gamma interferon further increased MDHM-mediated IL-6 release. Since monokines can be pyrogenic, we tested the effects of an intravenous injection of MDHM on rectal temperatures and leukocyte counts in rabbits. At 1 h after a bolus injection of MDHM, leukocyte counts dropped to about 35% of the initial values, reflecting a decrease in both lymphocytes and granulocytes. At 4 to 6 h after injection, granulocyte counts began to increase again, whereas lymphocyte counts remained low. No leukocytosis was noted during this time. The lack of leukocytosis can be explained by the failure of MDHM-stimulated macrophages to release IL-1. The property of MDHM to cause IL-6 release from macrophages and the IL-6 growth dependency of the 7TD1 hybridoma cell line were made use of in a coculture assay system to quantitate the activity of MDHM. With this method and macrophages from C3H/HeJ lipopolysaccharide-nonresponder mice, MDHM activity was found to be equally distributed in the mycoplasma growth medium and the sedimented mycoplasmas after sonication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Arenzana-Seisdedos F., Virelizier J. L., Fiers W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985 Apr;134(4):2444–2448. [PubMed] [Google Scholar]
- Atkin C. L., Cole B. C., Sullivan G. J., Washburn L. R., Wiley B. B. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. V. A small basic protein from culture supernatants is a potent T cell mitogen. J Immunol. 1986 Sep 1;137(5):1581–1589. [PubMed] [Google Scholar]
- Bauer J., Ganter U., Geiger T., Jacobshagen U., Hirano T., Matsuda T., Kishimoto T., Andus T., Acs G., Gerok W. Regulation of interleukin-6 expression in cultured human blood monocytes and monocyte-derived macrophages. Blood. 1988 Oct;72(4):1134–1140. [PubMed] [Google Scholar]
- Bredt W. Estimation of Mycoplasma pneumoniae inoculum size by rate of tetrazolium reduction. J Clin Microbiol. 1976 Jul;4(1):92–94. doi: 10.1128/jcm.4.1.92-94.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Marnoy S. O., Rosenwasser L. J. Role of arachidonate metabolism in the immunoregulatory function of human leukocytic pyrogen/lymphocyte-activating factor/interleukin 1. J Immunol. 1983 Feb;130(2):890–895. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Günther C., Röllinghoff M., Beuscher H. U. Proteolysis of the native murine IL 1 beta precursor is required to generate IL 1 beta bioactivity. Immunobiology. 1989 Feb;178(4-5):436–448. doi: 10.1016/s0171-2985(89)80064-6. [DOI] [PubMed] [Google Scholar]
- Hauschildt S., Wolf B., Lückhoff A., Bessler W. G. Determination of second messengers and protein kinase C in bone marrow derived macrophages stimulated with a bacterial lipopeptide. Mol Immunol. 1990 Jun;27(6):473–479. doi: 10.1016/0161-5890(90)90065-8. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Homfeld J., Homfeld A., Nicklas W., Rink L., Weyland A., Kirchner H. Induction of interleukin-6 in murine bone marrow-derived macrophages stimulated by the Mycoplasma arthritidis mitogen MAS. Autoimmunity. 1990;7(4):317–327. doi: 10.3109/08916939009087591. [DOI] [PubMed] [Google Scholar]
- Kampschmidt R. F., Upchurch H. F. Neutrophil release after injections of endotoxin or leukocytic endogenous mediator into rats. J Reticuloendothel Soc. 1980 Aug;28(2):191–201. [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosentreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by a macrophage cell line, P388D1. II. Biochemical characterization of LAF induced by activated T cells and LPS. J Immunol. 1978 May;120(5):1504–1508. [PubMed] [Google Scholar]
- Mühlradt P. F., Quentmeier H., Schmitt E. Involvement of interleukin-1 (IL-1), IL-6, IL-2, and IL-4 in generation of cytolytic T cells from thymocytes stimulated by a Mycoplasma fermentans-derived product. Infect Immun. 1991 Nov;59(11):3962–3968. doi: 10.1128/iai.59.11.3962-3968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., LeBlanc P. A., Murasko D. M. Comparative effects of various classes of mouse interferons on macrophage activation for tumor cell killing. J Immunol. 1985 Feb;134(2):977–981. [PubMed] [Google Scholar]
- Quentmeier H., Schmitt E., Kirchhoff H., Grote W., Mühlradt P. F. Mycoplasma fermentans-derived high-molecular-weight material induces interleukin-6 release in cultures of murine macrophages and human monocytes. Infect Immun. 1990 May;58(5):1273–1280. doi: 10.1128/iai.58.5.1273-1280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Sher T., Yamin A., Rottem S., Gallily R. In vitro induction of tumor necrosis factor alpha, tumor cytolysis, and blast transformation by Spiroplasma membranes. J Natl Cancer Inst. 1990 Jul 4;82(13):1142–1145. doi: 10.1093/jnci/82.13.1142. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Cassell G. H., Woodward J. G. Differential induction of bone marrow macrophage proliferation by mycoplasmas involves granulocyte-macrophage colony-stimulating factor. Infect Immun. 1990 Nov;58(11):3558–3563. doi: 10.1128/iai.58.11.3558-3563.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama K., Kuwano K., Furukawa M., Himeno Y., Satoh T., Arai S. Mycoplasmas induce transcription and production of tumor necrosis factor in a monocytic cell line, THP-1, by a protein kinase C-independent pathway. Infect Immun. 1990 Nov;58(11):3564–3567. doi: 10.1128/iai.58.11.3564-3567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Higuchi M., Sugimoto M., Shimoda O., Woo H. J., Shimada S., Osawa T. The production of a cytotoxic factor by mouse peritoneal macrophages and macrophage hybridomas treated with various stimulating agents. Microbiol Immunol. 1986;30(2):143–154. doi: 10.1111/j.1348-0421.1986.tb00929.x. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Keys M., Granger G. A., Ni R. X. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha-induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987 Nov 15;139(10):3406–3415. [PubMed] [Google Scholar]
- Van Damme J., De Ley M., Opdenakker G., Billiau A., De Somer P., Van Beeumen J. Homogeneous interferon-inducing 22K factor is related to endogenous pyrogen and interleukin-1. Nature. 1985 Mar 21;314(6008):266–268. doi: 10.1038/314266a0. [DOI] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]


