Abstract
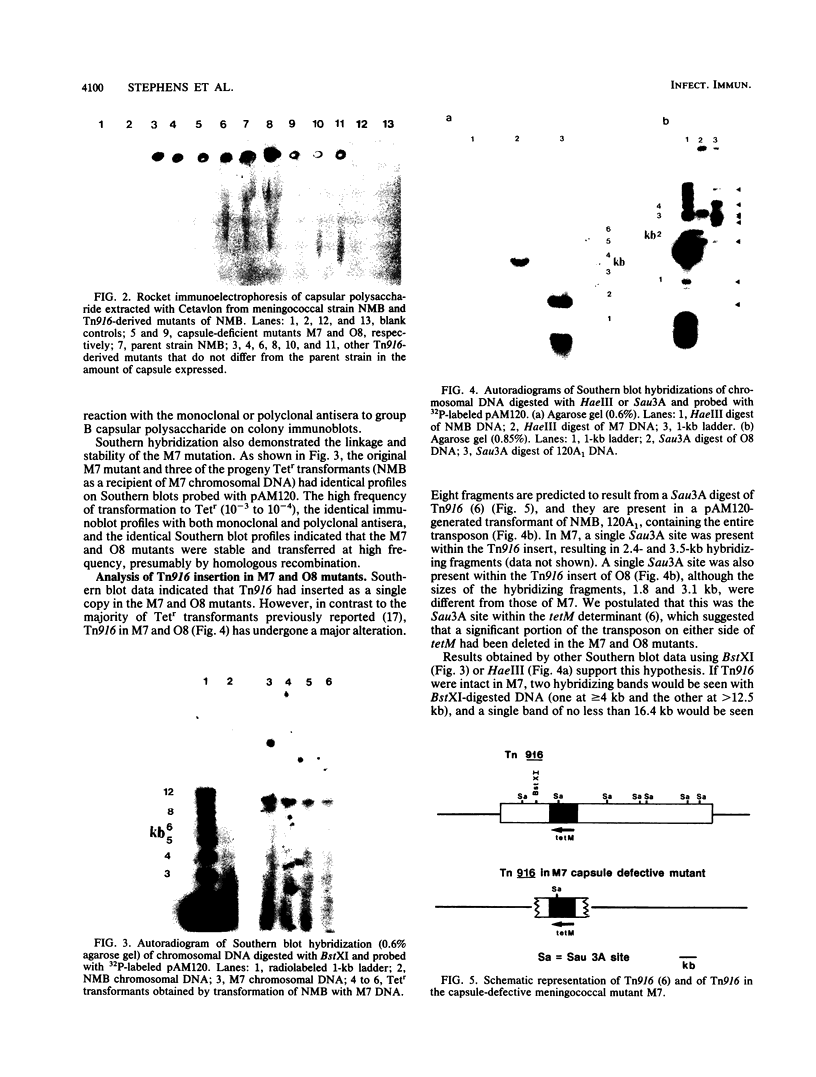
We recently found that the 16.4-kb conjugative transposon Tn916 could be introduced into Neisseria meningitidis by transformation and that it appeared to transpose to many different sites in the chromosome of recipient meningococci. In order to identify transposon-induced alterations of specific meningococcal virulence determinants, a library of meningococcal Tetr transformants containing Tn916 was made and screened for those altered in the production of group B capsular polysaccharide. A capsule-defective mutant, M7, was identified by using monoclonal and polyclonal antisera to group B polysaccharide in immunoblot and agar antiserum procedures. Growth of M7 was similar to that of the parent strain. M7 produced no group B capsular polysaccharide by rocket immunoelectrophoresis, and the mutation was stable during laboratory passage. The capsule-defective phenotype was linked to Tetr, as demonstrated by immunoblot and Southern blot analysis of progeny Tetr transformants (transformants of the parent strain obtained with DNA from M7). A capsule-deficient mutant, O8, was identified by using a similar approach. Analysis of the Tn916 insertions in M7 and O8 indicated that a significant portion of the transposon on either side of the tetM determinant had been lost. The ability of Tn916 to generate defined, stable mutations in meningococcal virulence determinants is demonstrated by our study.
Full text
PDF

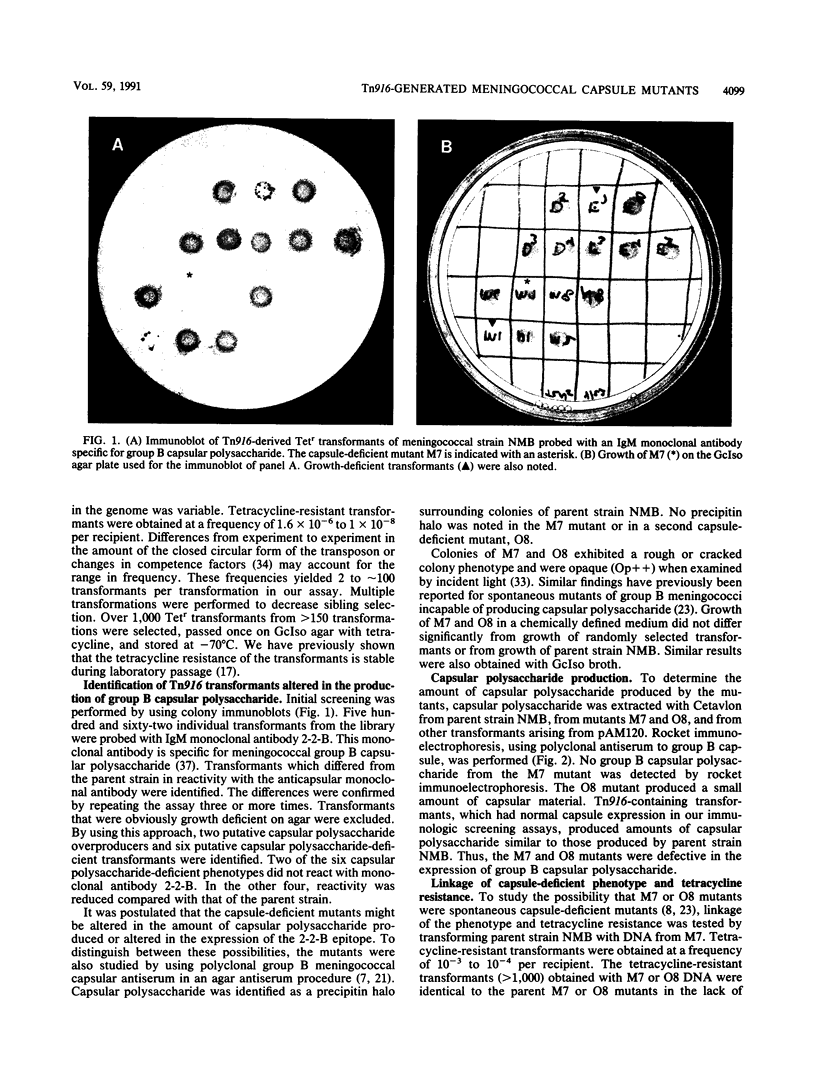


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. E., Iandolo J. J., Campbell B. S., Davidson L. I., Bulla L. A. Rocket Immunoelectrophoresis of the Entomocidal Parasporal Crystal of Bacillus thuringiensis subsp. kurstaki. Appl Environ Microbiol. 1980 Nov;40(5):897–900. doi: 10.1128/aem.40.5.897-900.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud F., Courvalin P. Nucleotide sequence of the ends of the conjugative shuttle transposon Tn1545. Mol Gen Genet. 1987 Aug;209(1):110–115. doi: 10.1007/BF00329844. [DOI] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell. 1989 Dec 22;59(6):1027–1034. doi: 10.1016/0092-8674(89)90759-9. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Flannagan S. E., Ike Y., Jones J. M., Gawron-Burke C. Sequence analysis of termini of conjugative transposon Tn916. J Bacteriol. 1988 Jul;170(7):3046–3052. doi: 10.1128/jb.170.7.3046-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Frasch C. E., Robbins J. B., Feldman H. A. Serogroup identification of Neisseria meningitidis: comparison of an antiserum agar method with bacterial slide agglutination. J Clin Microbiol. 1978 May;7(5):410–414. doi: 10.1128/jcm.7.5.410-414.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine L. F., Rhode S. L., 3rd, Hagerman C. R. Relationship of serogroups of Neisseria meningitidis. II. R variants of Neisseria meningitidis. Infect Immun. 1972 Jan;5(1):48–54. doi: 10.1128/iai.5.1.48-54.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson C. G., Jephcott A. E., Gough K. R., Spratt B. G. Penicillin-binding protein 2 genes of non-beta-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1989 Jan;3(1):35–41. doi: 10.1111/j.1365-2958.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frosch M., Schultz E., Glenn-Calvo E., Meyer T. F. Generation of capsule-deficient Neisseria meningitidis strains by homologous recombination. Mol Microbiol. 1990 Jul;4(7):1215–1218. doi: 10.1111/j.1365-2958.1990.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Frosch M., Weisgerber C., Meyer T. F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D. L., Jones K. R., Tobian J. A., LeBlanc D. J., Macrina F. L. Disseminated tetracycline resistance in oral streptococci: implication of a conjugative transposon. Infect Immun. 1984 Jul;45(1):13–17. doi: 10.1128/iai.45.1.13-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hächler H., Kayser F. H., Berger-Bächi B. Homology of a transferable tetracycline resistance determinant of Clostridium difficile with Streptococcus (Enterococcus) faecalis transposon Tn916. Antimicrob Agents Chemother. 1987 Jul;31(7):1033–1038. doi: 10.1128/aac.31.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik A., Juni E., Heym G. A. Genetic Transformation as a tool for detection of Neisseria gonorrhoeae. J Clin Microbiol. 1976 Jul;4(1):71–81. doi: 10.1128/jcm.4.1.71-81.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Stephens D. S., Spellman P., Morse S. A. Transposition of Tn916 to different sites in the chromosome of Neisseria meningitidis: a genetic tool for meningococcal mutagenesis. Mol Microbiol. 1990 May;4(5):729–735. doi: 10.1111/j.1365-2958.1990.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Kauc L., Goodgal S. H. Introduction of transposon Tn916 DNA into Haemophilus influenzae and Haemophilus parainfluenzae. J Bacteriol. 1989 Dec;171(12):6625–6628. doi: 10.1128/jb.171.12.6625-6628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Johnson S. R., Zenilman J. M., Roberts M. C., Morse S. A. High-level tetracycline resistance resulting from TetM in strains of Neisseria spp., Kingella denitrificans, and Eikenella corrodens. Antimicrob Agents Chemother. 1988 May;32(5):765–767. doi: 10.1128/aac.32.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Zenilman J. M., Biddle J. W., Perkins G. H., DeWitt W. E., Thomas M. L., Johnson S. R., Morse S. A. Frequency and distribution in the United States of strains of Neisseria gonorrhoeae with plasmid-mediated, high-level resistance to tetracycline. J Infect Dis. 1987 Apr;155(4):819–822. doi: 10.1093/infdis/155.4.819. [DOI] [PubMed] [Google Scholar]
- Kuzemenská P., Burian V., Frasch C. E., Svandová E. A comparison of the serum agar grouping and slide agglutination methods for the identification of Neisseria meningitidis strains. Bull World Health Organ. 1977;55(6):659–661. [PMC free article] [PubMed] [Google Scholar]
- Martin P., Trieu-Cuot P., Courvalin P. Nucleotide sequence of the tetM tetracycline resistance determinant of the streptococcal conjugative shuttle transposon Tn1545. Nucleic Acids Res. 1986 Sep 11;14(17):7047–7058. doi: 10.1093/nar/14.17.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Johnson S. R., Biddle J. W., Roberts M. C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986 Nov;30(5):664–670. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X., Puaoi D., So M. Transposition of Tn1545-delta 3 in the pathogenic Neisseriae: a genetic tool for mutagenesis. J Bacteriol. 1991 Apr;173(7):2147–2154. doi: 10.1128/jb.173.7.2147-2154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. Molecular characterization of two proteins involved in the excision of the conjugative transposon Tn1545: homologies with other site-specific recombinases. EMBO J. 1989 Aug;8(8):2425–2433. doi: 10.1002/j.1460-2075.1989.tb08373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Kirchman P. A., Caparon M. G. An intermediate in transposition of the conjugative transposon Tn916. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4809–4813. doi: 10.1073/pnas.85.13.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senghas E., Jones J. M., Yamamoto M., Gawron-Burke C., Clewell D. B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988 Jan;170(1):245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., Farley M. M. Pathogenic events during infection of the human nasopharynx with Neisseria meningitidis and Haemophilus influenzae. Rev Infect Dis. 1991 Jan-Feb;13(1):22–33. doi: 10.1093/clinids/13.1.22. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Association of virulence of Neisseria meningitidis with transparent colony type and low-molecular-weight outer membrane proteins. J Infect Dis. 1983 Feb;147(2):282–292. doi: 10.1093/infdis/147.2.282. [DOI] [PubMed] [Google Scholar]
- West S. E., Clark V. L. Genetic loci and linkage associations in Neisseria gonorrhoeae and Neisseria meningitidis. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S92–103. doi: 10.1128/cmr.2.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]