Abstract
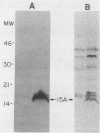
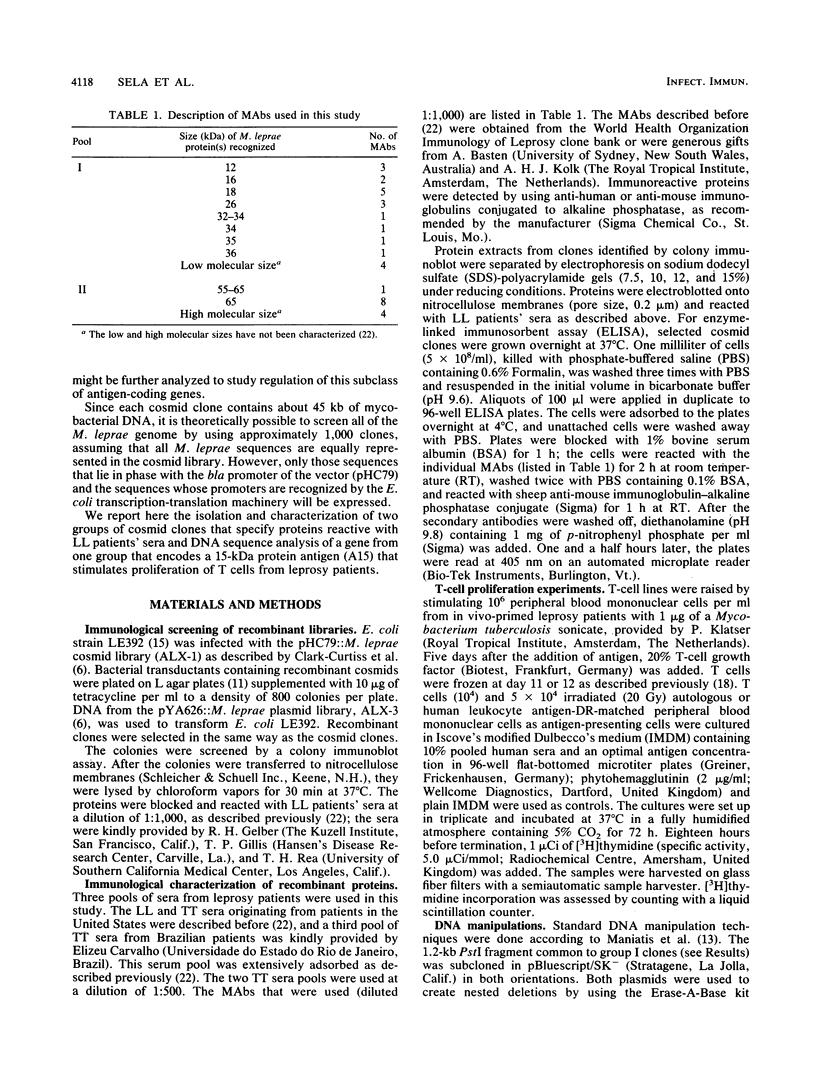
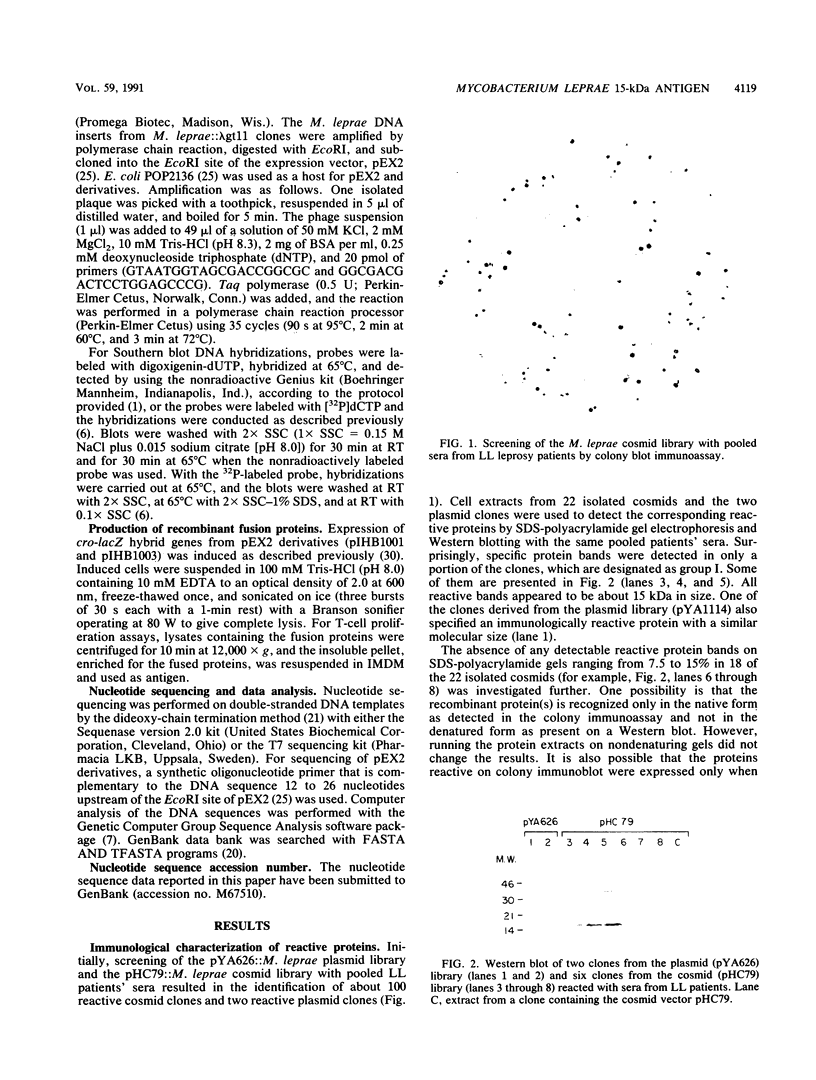
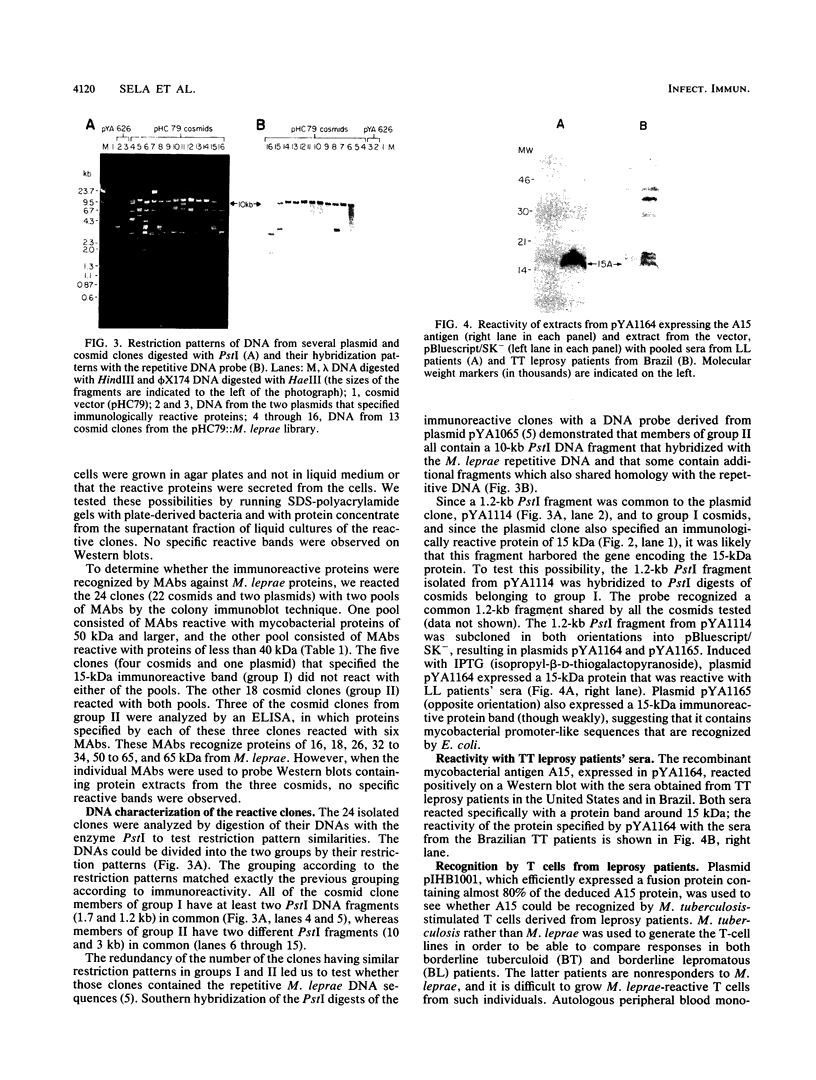
Screening of the Mycobacterium leprae cosmid library with pooled sera from lepromatous leprosy (LL) patients by a colony immunoblot technique resulted in the identification of about 100 colonies that produced immunologically reactive proteins. Twenty-four of these clones were purified, analyzed, and found to comprise two groups according to the reactivity of the recombinant proteins with LL sera and to the DNA restriction patterns of the recombinant plasmids and cosmids. Proteins specified by clones from group I reacted strongly with LL patients' sera on a Western blot (immunoblot), demonstrating a 15-kDa protein band designated A15. The A15 antigen also reacted with pooled sera from patients with tuberculoid leprosy from the United States and Brazil. Clones from group II did not show any reactive protein band on a Western blot, when reacted with patients' sera. DNAs from cosmids of group II all contain a 10-kb PstI fragment that hybridized to the unique repetitive M. leprae DNA. Sequence analysis of a 1.2-kb fragment containing the entire coding sequence of A15 revealed three open reading frames (ORFs), only one of which (ORF II) contains sufficient genetic information to encode for A15. Part of the A15 gene was found to exist also in a group of lambda gt11:M. leprae clones previously isolated in our laboratory by immunological screening with LL patients' sera. One of the lambda gt11 clones (L8) expresses a beta-galactosidase fusion protein with 89 amino acids from the C terminus of A15. An important result was that the fusion protein was clearly recognized by T cells from leprosy patients. Interestingly, Mycobacterium tuberculosis-stimulated T cells from M. leprae nonresponder (LL as well as borderline tuberculoid) patients were able to respond to the isolated recombinant M. leprae antigen, indicating that nonresponsiveness to M. leprae antigens can be reversible. The sequence of the M. leprae DNA fused to the beta-galactosidase gene of lambda gt11 clone L8 was identical to that of a lambda gt11:M. leprae clone isolated recently that expresses an immunologically reactive fusion protein (S. Laal, Y. D. Sharma, H. K. Prasad, A. Murtaza, S. Singh, S. Tangri, R. Misra, and I. Nath, Proc. Natl. Acad. Sci. USA 88:1054-1058, 1991). Besides the complete sequence of the A15 gene, sequencing data of two flanking ORFs are presented. Downstream from ORF II (A15), ORF III has a high degree of similarity to the genes for tomato ATP-dependent proteases that are members of a larger class of highly conserved proteases ubiquitous among prokaryotes and eukaryotes.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

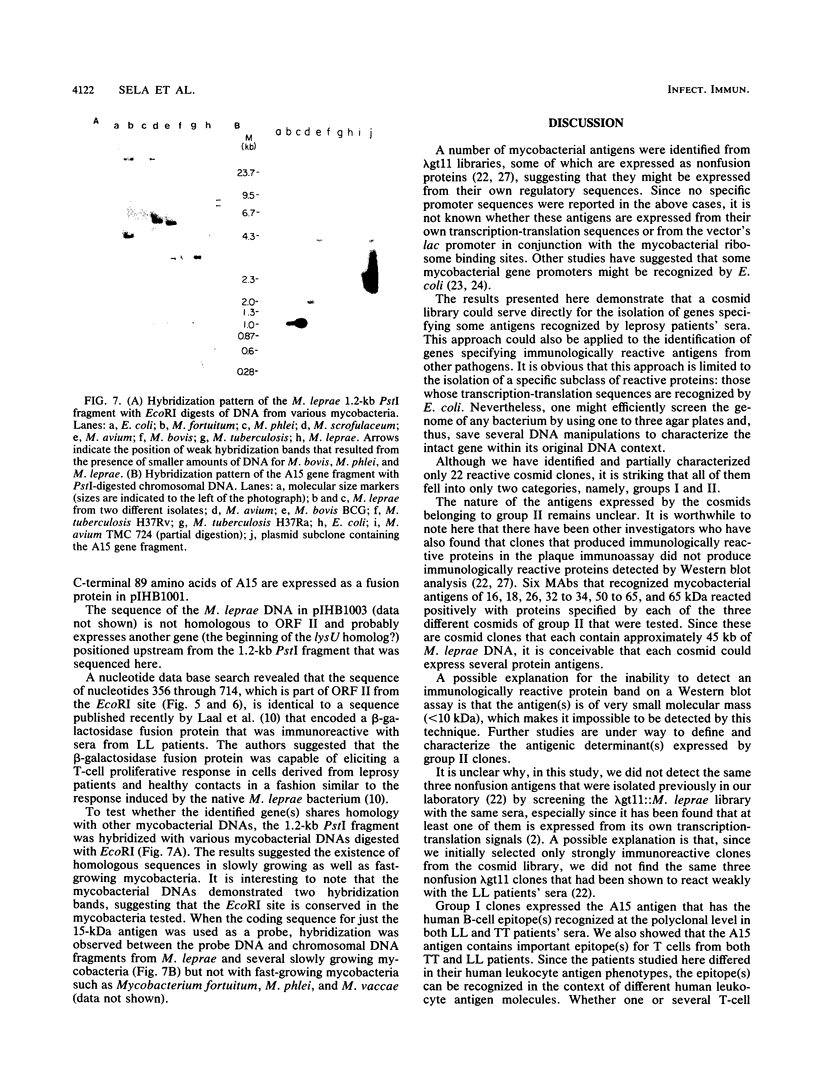


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherayil B. J., Young R. A. A 28-kDa protein from Mycobacterium leprae is a target of the human antibody response in lepromatous leprosy. J Immunol. 1988 Dec 15;141(12):4370–4375. [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. L., Neidhardt F. C. Roles of the two lysyl-tRNA synthetases of Escherichia coli: analysis of nucleotide sequences and mutant behavior. J Bacteriol. 1990 Jun;172(6):3237–3243. doi: 10.1128/jb.172.6.3237-3243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsia R. J., Hellqvist L., Booth R. J., Radford A. J., Britton W. J., Astbury L., Trent R. J., Basten A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect Immun. 1989 Jan;57(1):204–212. doi: 10.1128/iai.57.1.204-212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Squires C., Pichersky E., Carrington M., Hobbs M., Mattick J. S., Dalrymple B., Kuramitsu H., Shiroza T., Foster T. Conservation of the regulatory subunit for the Clp ATP-dependent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci U S A. 1990 May;87(9):3513–3517. doi: 10.1073/pnas.87.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laal S., Sharma Y. D., Prasad H. K., Murtaza A., Singh S., Tangri S., Misra R. S., Nath I. Recombinant fusion protein identified by lepromatous sera mimics native Mycobacterium leprae in T-cell responses across the leprosy spectrum. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1054–1058. doi: 10.1073/pnas.88.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévêque F., Plateau P., Dessen P., Blanquet S. Homology of lysS and lysU, the two Escherichia coli genes encoding distinct lysyl-tRNA synthetase species. Nucleic Acids Res. 1990 Jan 25;18(2):305–312. doi: 10.1093/nar/18.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Converse P. J., Gebre N., Wondimu A., Ehrenberg J. P., Kiessling R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur J Immunol. 1989 Apr;19(4):707–713. doi: 10.1002/eji.1830190421. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Elferink D. G., Hermans J., de Vries R. R. HLA class II restriction repertoire of antigen-specific T cells. I. The main restriction determinants for antigen presentation are associated with HLA-D/DR and not with DP and DQ. Hum Immunol. 1985 Jun;13(2):105–116. doi: 10.1016/0198-8859(85)90017-5. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Wondimu A., Reddy N. N. A comparative study on the effects of rIL-4, rIL-2, rIFN-gamma, and rTNF-alpha on specific T-cell non-responsiveness to mycobacterial antigens in lepromatous leprosy patients in vitro. Scand J Immunol. 1990 May;31(5):553–565. doi: 10.1111/j.1365-3083.1990.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish M., Esser R. E., Thole J. E., Clark-Curtiss J. E. Identification and characterization of antigenic determinants of Mycobacterium leprae that react with antibodies in sera of leprosy patients. Infect Immun. 1990 May;58(5):1327–1336. doi: 10.1128/iai.58.5.1327-1336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela S., Clark-Curtiss J. E. Cloning and characterization of the Mycobacterium leprae putative ribosomal RNA promoter in Escherichia coli. Gene. 1991 Feb 1;98(1):123–127. doi: 10.1016/0378-1119(91)90114-q. [DOI] [PubMed] [Google Scholar]
- Sirakova T. D., Bardarov S. S., Kriakov J. I., Markov K. I. Molecular cloning of mycobacterial promoters in Escherichia coli. FEMS Microbiol Lett. 1989 May;50(1-2):153–156. doi: 10.1016/0378-1097(89)90476-x. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Stabel L. F., Suykerbuyk M. E., De Wit M. Y., Klatser P. R., Kolk A. H., Hartskeerl R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an immunoreactive region of proline-rich repeats. Infect Immun. 1990 Jan;58(1):80–87. doi: 10.1128/iai.58.1.80-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kent L., Young R. A. Screening of a recombinant mycobacterial DNA library with polyclonal antiserum and molecular weight analysis of expressed antigens. Infect Immun. 1987 Jun;55(6):1421–1425. doi: 10.1128/iai.55.6.1421-1425.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]