Abstract
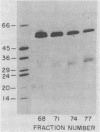
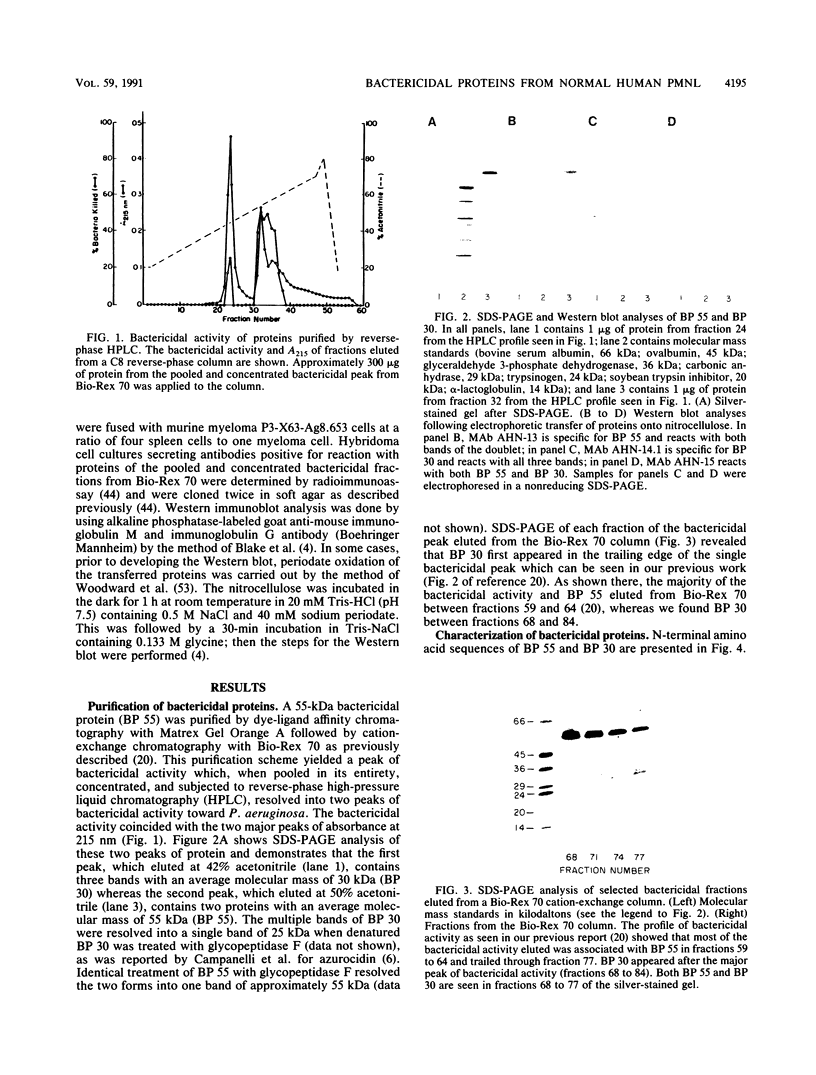
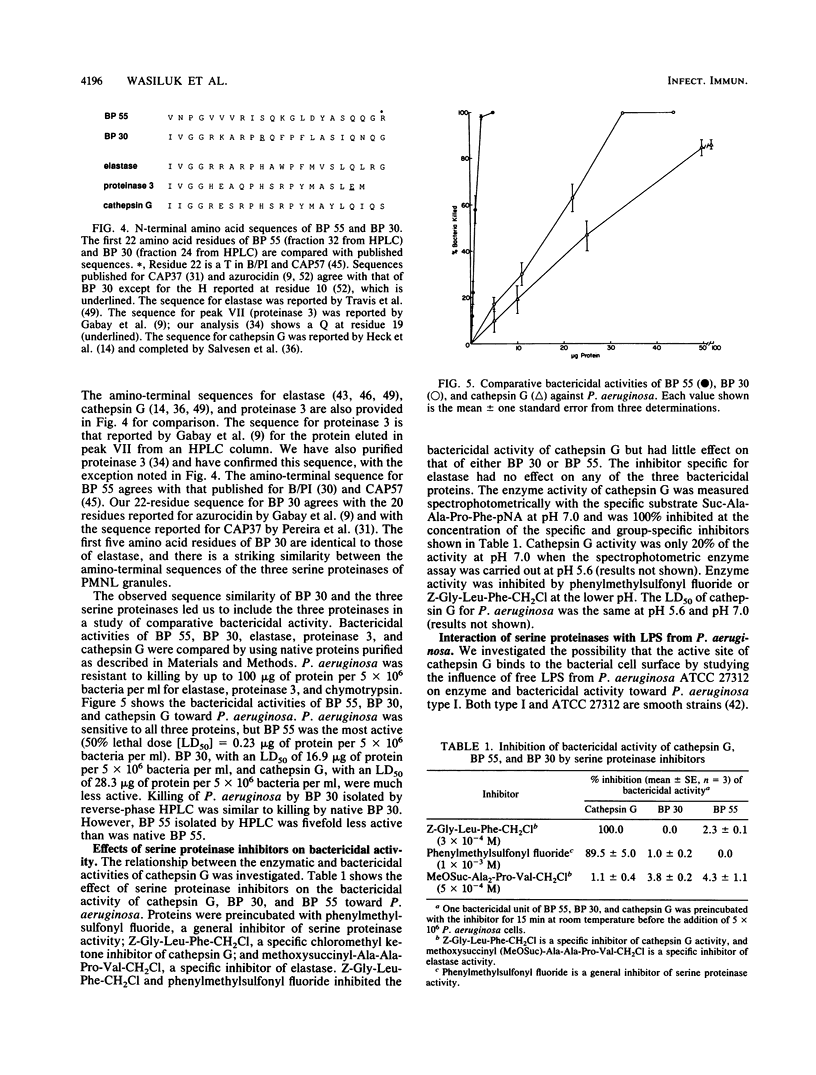
Killing of Pseudomonas aeruginosa by a 55-kDa bactericidal protein (BP 55), a 30-kDa protein (BP 30), cathepsin G, elastase, and proteinase 3 has been compared. P. aeruginosa was resistant to killing by elastase and proteinase 3. BP 55 at a 50% lethal dose (LD50) of 0.23 micrograms of protein per 5 x 10(6) bacteria per ml killed P. aeruginosa and was far more active than BP 30 and cathepsin G. The LD50s of BP 30 and cathepsin G were 16.9 and 28.3 micrograms of protein per 5 x 10(6) bacteria per ml, respectively. Preincubation of BP 55 or BP 30 with lipopolysaccharide (LPS) from P. aeruginosa inhibited bactericidal activity. The N-terminal amino acid sequence of BP 55 and BP 30 revealed no relationship between the two proteins. However, a monoclonal antibody (AHN-15) reacted with both proteins by Western immunoblot. The bactericidal activity of cathepsin G toward P. aeruginosa appeared to be dependent on the availability of the active site of the enzyme; bactericidal activity was inhibited by phenylmethylsulfonyl fluoride (PMSF) and by the specific cathepsin G inhibitor, Z-Gly-Leu-Phe-CH2Cl. The enzyme and bactericidal activities of cathepsin G were also inhibited by LPS from P. aeruginosa. LPS from P. aeruginosa was shown to be a competitive inhibitor of the enzyme activity of cathepsin G. Elastase enzyme activity was also inhibited noncompetitively by LPS, but the enzyme was not bactericidal. We have concluded that all three bactericidal proteins (BP 55, BP 30, and cathepsin G) may bind to the LPS of the outer membrane of P. aeruginosa. It appears that the enzyme active site must be available for cathepsin G to kill P. aeruginosa and that the active site may be involved in the binding of cathepsin G to P. aeruginosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford C. E., Amaral E., Campbell P. A. Listericidal activity of human neutrophil cathepsin G. J Gen Microbiol. 1990 Jun;136(6):997–100. doi: 10.1099/00221287-136-6-997. [DOI] [PubMed] [Google Scholar]
- Ashe B. M., Zimmerman M. Specific inhibition of human granulocyte elastase by cis-unsaturated fatty acids and activation by the corresponding alcohols. Biochem Biophys Res Commun. 1977 Mar 7;75(1):194–199. doi: 10.1016/0006-291x(77)91308-0. [DOI] [PubMed] [Google Scholar]
- Bangalore N., Travis J., Onunka V. C., Pohl J., Shafer W. M. Identification of the primary antimicrobial domains in human neutrophil cathepsin G. J Biol Chem. 1990 Aug 15;265(23):13584–13588. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanelli D., Detmers P. A., Nathan C. F., Gabay J. E. Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Invest. 1990 Mar;85(3):904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Scott R. W., Campanelli D., Griffith J., Wilde C., Marra M. N., Seeger M., Nathan C. F. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Flaggs G., Leong S. R., Gumina R. J., Weiss J., Ooi C. E., Elsbach P. Cloning of the cDNA of a human neutrophil bactericidal protein. Structural and functional correlations. J Biol Chem. 1989 Jun 5;264(16):9505–9509. [PubMed] [Google Scholar]
- Hameed A., Lowrey D. M., Lichtenheld M., Podack E. R. Characterization of three serine esterases isolated from human IL-2 activated killer cells. J Immunol. 1988 Nov 1;141(9):3142–3147. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Heck L. W., Rostand K. S., Hunter F. A., Bhown A. Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil cathepsin G from normal donors. Anal Biochem. 1986 Oct;158(1):217–227. doi: 10.1016/0003-2697(86)90612-3. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hill C. P., Yee J., Selsted M. E., Eisenberg D. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science. 1991 Mar 22;251(5000):1481–1485. doi: 10.1126/science.2006422. [DOI] [PubMed] [Google Scholar]
- Hovde C. J., Gray B. H. Characterization of a protein from normal human polymorphonuclear leukocytes with bactericidal activity against Pseudomonas aeruginosa. Infect Immun. 1986 Oct;54(1):142–148. doi: 10.1128/iai.54.1.142-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovde C. J., Gray B. H. Physiological effects of a bactericidal protein from human polymorphonuclear leukocytes on Pseudomonas aeruginosa. Infect Immun. 1986 Apr;52(1):90–95. doi: 10.1128/iai.52.1.90-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao R. C., Wehner N. G., Skubitz K. M., Gray B. H., Hoidal J. R. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest. 1988 Dec;82(6):1963–1973. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leytus S. P., Loeb K. R., Hagen F. S., Kurachi K., Davie E. W. A novel trypsin-like serine protease (hepsin) with a putative transmembrane domain expressed by human liver and hepatoma cells. Biochemistry. 1988 Feb 9;27(3):1067–1074. doi: 10.1021/bi00403a032. [DOI] [PubMed] [Google Scholar]
- Marra M. N., Wilde C. G., Griffith J. E., Snable J. L., Scott R. W. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J Immunol. 1990 Jan 15;144(2):662–666. [PubMed] [Google Scholar]
- Miyasaki K. T., Bodeau A. L. In vitro killing of oral Capnocytophaga by granule fractions of human neutrophils is associated with cathepsin G activity. J Clin Invest. 1991 May;87(5):1585–1593. doi: 10.1172/JCI115172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J., Elsbach P., Frangione B., Mannion B. A 25-kDa NH2-terminal fragment carries all the antibacterial activities of the human neutrophil 60-kDa bactericidal/permeability-increasing protein. J Biol Chem. 1987 Nov 5;262(31):14891–14894. [PubMed] [Google Scholar]
- Pereira H. A., Spitznagel J. K., Pohl J., Wilson D. E., Morgan J., Palings I., Larrick J. W. CAP 37, a 37 kD human neutrophil granule cationic protein shares homology with inflammatory proteinases. Life Sci. 1990;46(3):189–196. doi: 10.1016/0024-3205(90)90104-y. [DOI] [PubMed] [Google Scholar]
- Pereira H. A., Spitznagel J. K., Winton E. F., Shafer W. M., Martin L. E., Guzman G. S., Pohl J., Scott R. W., Marra M. N., Kinkade J. M., Jr The ontogeny of a 57-Kd cationic antimicrobial protein of human polymorphonuclear leukocytes: localization to a novel granule population. Blood. 1990 Aug 15;76(4):825–834. [PubMed] [Google Scholar]
- Pohl J., Pereira H. A., Martin N. M., Spitznagel J. K. Amino acid sequence of CAP37, a human neutrophil granule-derived antibacterial and monocyte-specific chemotactic glycoprotein structurally similar to neutrophil elastase. FEBS Lett. 1990 Oct 15;272(1-2):200–204. doi: 10.1016/0014-5793(90)80484-z. [DOI] [PubMed] [Google Scholar]
- Rao N. V., Wehner N. G., Marshall B. C., Gray W. R., Gray B. H., Hoidal J. R. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J Biol Chem. 1991 May 25;266(15):9540–9548. [PubMed] [Google Scholar]
- Salvesen G., Farley D., Shuman J., Przybyla A., Reilly C., Travis J. Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry. 1987 Apr 21;26(8):2289–2293. doi: 10.1021/bi00382a032. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V. C., Jannoun M., Huthwaite L. W. Molecular mechanism for the antigonococcal action of lysosomal cathepsin G. Mol Microbiol. 1990 Aug;4(8):1269–1277. doi: 10.1111/j.1365-2958.1990.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V. C., Martin L. E. Antigonococcal activity of human neutrophil cathepsin G. Infect Immun. 1986 Oct;54(1):184–188. doi: 10.1128/iai.54.1.184-188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V. C. Mechanism of staphylococcal resistance to non-oxidative antimicrobial action of neutrophils: importance of pH and ionic strength in determining the bactericidal action of cathepsin G. J Gen Microbiol. 1989 Apr;135(4):825–830. doi: 10.1099/00221287-135-4-825. [DOI] [PubMed] [Google Scholar]
- Siefferman C. M., Regelmann W. E., Gray B. H. Pseudomonas aeruginosa variants isolated from patients with cystic fibrosis are killed by a bactericidal protein from human polymorphonuclear leukocytes. Infect Immun. 1991 Jun;59(6):2152–2157. doi: 10.1128/iai.59.6.2152-2157.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Watorek W., Karr S., Giles J., Bode W., Travis J. Primary structure of human neutrophil elastase. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2228–2232. doi: 10.1073/pnas.84.8.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz K. M., Zhen Y., August J. T. A human granulocyte-specific antigen characterized by use of monoclonal antibodies. Blood. 1983 Jan;61(1):19–26. [PubMed] [Google Scholar]
- Spitznagel J. K. Antibiotic proteins of human neutrophils. J Clin Invest. 1990 Nov;86(5):1381–1386. doi: 10.1172/JCI114851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Nukiwa T., Yoshimura K., Quick C. D., States D. J., Holmes M. D., Whang-Peng J., Knutsen T., Crystal R. G. Structure of the human neutrophil elastase gene. J Biol Chem. 1988 Oct 15;263(29):14739–14747. [PubMed] [Google Scholar]
- Thomas E. L., Lehrer R. I., Rest R. F. Human neutrophil antimicrobial activity. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S450–S456. doi: 10.1093/cid/10.supplement_2.s450. [DOI] [PubMed] [Google Scholar]
- Thomas J. M., Hodes M. E. A new discontinuous buffer system for the electrophoresis of cationic proteins at near-neutral pH. Anal Biochem. 1981 Nov 15;118(1):194–196. doi: 10.1016/0003-2697(81)90178-0. [DOI] [PubMed] [Google Scholar]
- Travis J., Giles P. J., Porcelli L., Reilly C. F., Baugh R., Powers J. Human leucocyte elastase and cathepsin G: structural and functional characteristics. Ciba Found Symp. 1979;(75):51–68. doi: 10.1002/9780470720585.ch4. [DOI] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Wilde C. G., Snable J. L., Griffith J. E., Scott R. W. Characterization of two azurphil granule proteases with active-site homology to neutrophil elastase. J Biol Chem. 1990 Feb 5;265(4):2038–2041. [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]