Abstract
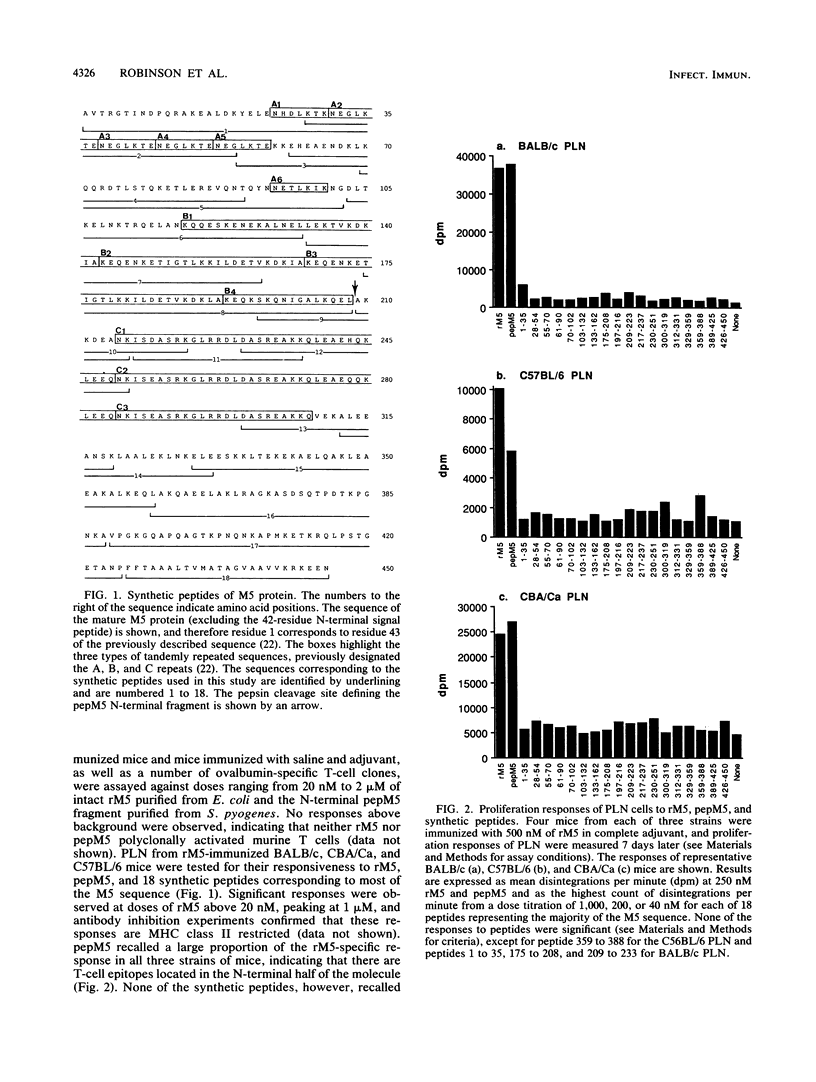
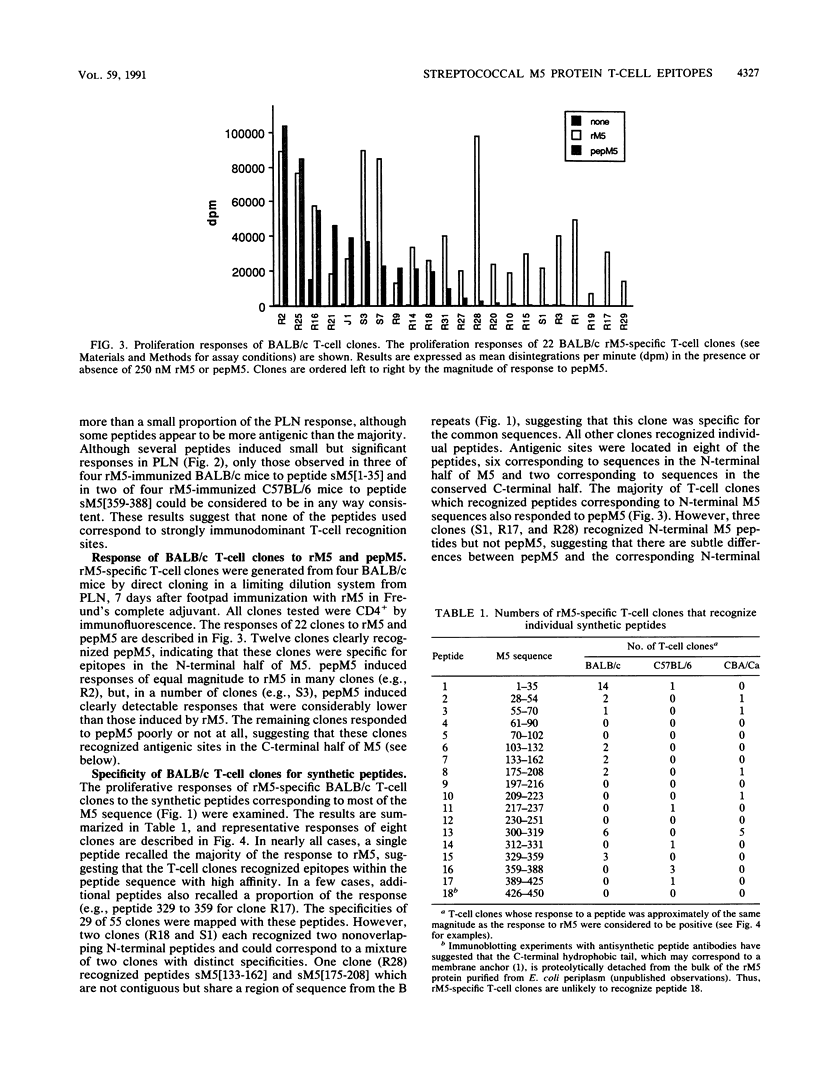
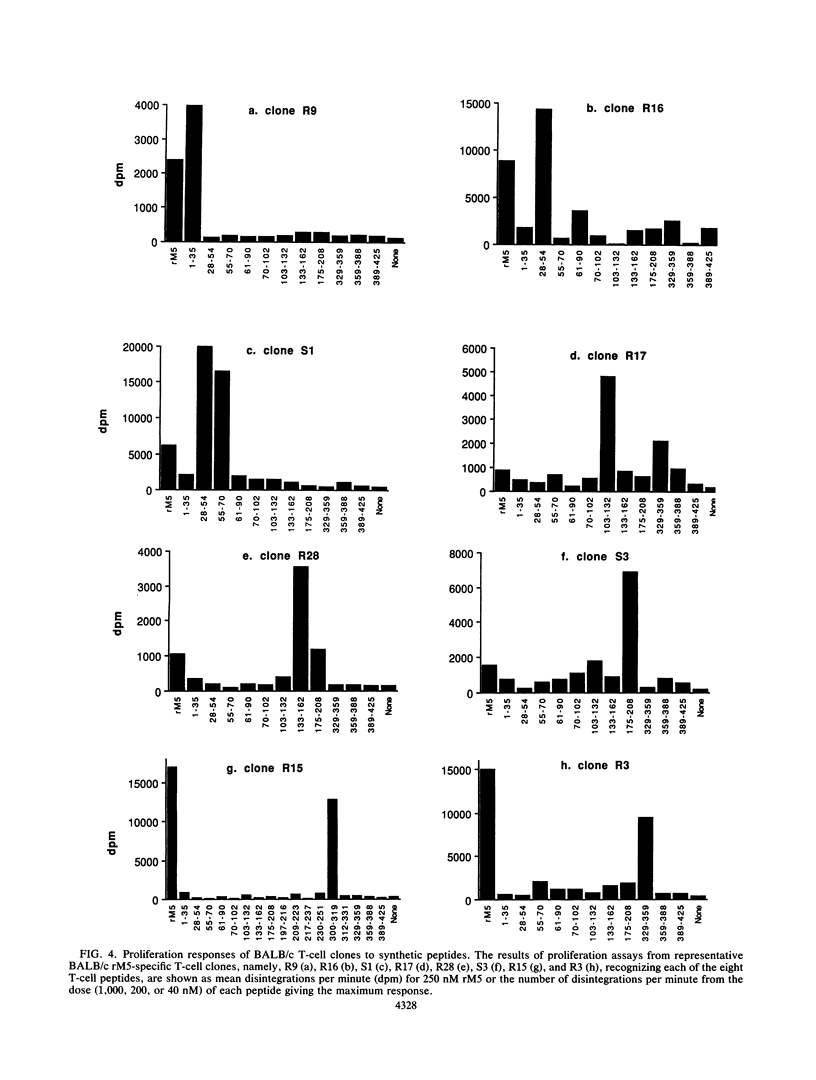
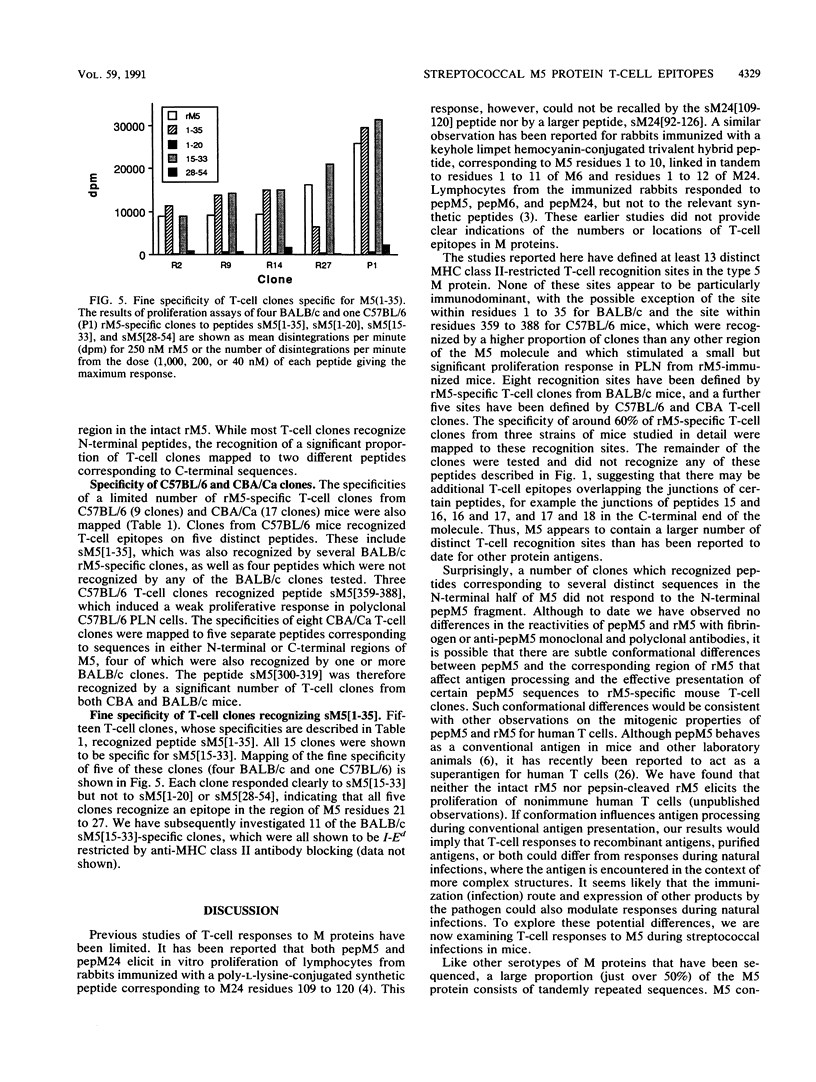
Group A streptococcal cell surface M proteins elicit highly protective, serotype-specific opsonic antibodies and many serotypes also elicit host cross-reactive antibodies, which may contribute to the pathogenesis of poststreptococcal autoimmune disease. To date, studies aimed at designing safe (non-host-cross-reactive, defined-epitope) M vaccines have focused almost exclusively on antibody epitopes. Here we identify T-cell epitopes recognized by T cells from BALB/c, C57BL/6, and CBA/Ca mice immunized with purified, recombinant serotype 5 M protein (rM5). The responses of rM5-specific, major histocompatibility complex class II-restricted, T-cell clones to synthetic peptides representing most of the M5 sequence identified at least 13 distinct T-cell recognition sites, including sites recognized by more than one major histocompatibility complex haplotype of mice. Although none of these sites appeared to be strongly immunodominant, an N-terminal peptide, sM5[1-35], was recognized by lymph node T cells of rM5-immunized mice and by a larger proportion of rM5-specific T-cell clones than any other individual peptide. The fine specificity of these clones was mapped with subpeptides to a single site at or overlapping the sequence ELENHDL at residues 21 to 27, which is in close proximity to previously mapped protective antibody epitopes. Other T-cell recognition sites are distributed throughout the M protein and include several in the highly conserved C-terminal region of the molecule. One of these C-terminal sites, located within residues 300 to 319, was recognized by a significant proportion of T-cell clones from two strains of mice. Helper T-cell epitopes located in the C-terminal region of M5 are likely to be widely conserved between different M serotypes and could be particularly useful in designing multivalent, defined-epitope M vaccines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett B. C., Burt D. S., Graham C. M., Warren A. P., Skehel J. J., Thomas D. B. I-Ad restricted T cell recognition of influenza hemagglutinin. Synthetic peptides identify multiple epitopes corresponding to antibody-binding regions of the HA1 subunit. J Immunol. 1989 Oct 15;143(8):2663–2669. [PubMed] [Google Scholar]
- Beachey E. H., Campbell G. L., Ofek I. Peptic digestion of streptococcal M protein. II. Extraction of M antigen from group A streptococci with pepsin. Infect Immun. 1974 May;9(5):891–896. doi: 10.1128/iai.9.5.891-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Dale J. B. Protective immunogenicity and T lymphocyte specificity of a trivalent hybrid peptide containing NH2-terminal sequences of types 5, 6, and 24 M proteins synthesized in tandem. J Exp Med. 1987 Sep 1;166(3):647–656. doi: 10.1084/jem.166.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Dale J. B., Simpson W. A., Kang A. H. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature. 1981 Jul 30;292(5822):457–459. doi: 10.1038/292457a0. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Structural features of protein antigenic sites recognized by helper T cells: what makes a site immunodominant? Year Immunol. 1986;2:28–38. [PubMed] [Google Scholar]
- Dale J. B., Simpson W. A., Ofek I., Beachey E. H. Blastogenic responses of human lymphocytes to structurally defined polypeptide fragments of streptococcal M protein. J Immunol. 1981 Apr;126(4):1499–1505. [PubMed] [Google Scholar]
- Finnegan A., Smith M. A., Smith J. A., Berzofsky J., Sachs D. H., Hodes R. J. The T cell repertoire for recognition of a phylogenetically distant protein antigen. Peptide specificity and MHC restriction of staphylococcal nuclease-specific T cell clones. J Exp Med. 1986 Sep 1;164(3):897–910. doi: 10.1084/jem.164.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Windels M. Mapping the immunodeterminants of the complete streptococcal M6 protein molecule. Identification of an immunodominant region. J Immunol. 1988 Nov 15;141(10):3592–3599. [PubMed] [Google Scholar]
- Gammon G., Geysen H. M., Apple R. J., Pickett E., Palmer M., Ametani A., Sercarz E. E. T cell determinant structure: cores and determinant envelopes in three mouse major histocompatibility complex haplotypes. J Exp Med. 1991 Mar 1;173(3):609–617. doi: 10.1084/jem.173.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. M., Liew F. Y., Tite J. P. Identification and characterization of T helper epitopes in the nucleoprotein of influenza A virus. J Immunol. 1989 Nov 1;143(9):3007–3014. [PubMed] [Google Scholar]
- Good M. F., Pombo D., Quakyi I. A., Riley E. M., Houghten R. A., Menon A., Alling D. W., Berzofsky J. A., Miller L. H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale P. M., Cease K. B., Houghten R. A., Ouyang C., Putney S., Javaherian K., Margalit H., Cornette J. L., Spouge J. L., DeLisi C. T cell multideterminant regions in the human immunodeficiency virus envelope: toward overcoming the problem of major histocompatibility complex restriction. Int Immunol. 1989;1(4):409–415. doi: 10.1093/intimm/1.4.409. [DOI] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Kehoe M. A., Poirier T. P., Beachey E. H., Timmis K. N. Cloning and genetic analysis of serotype 5 M protein determinant of group A streptococci: evidence for multiple copies of the M5 determinant in the Streptococcus pyogenes genome. Infect Immun. 1985 Apr;48(1):190–197. doi: 10.1128/iai.48.1.190-197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone A. M., Fathman C. G. The structure of T-cell epitopes. Annu Rev Immunol. 1987;5:477–501. doi: 10.1146/annurev.iy.05.040187.002401. [DOI] [PubMed] [Google Scholar]
- Miller L., Gray L., Beachey E., Kehoe M. Antigenic variation among group A streptococcal M proteins. Nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988 Apr 25;263(12):5668–5673. [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Scherer M. T., Briner T. J., Smith J. A., Gefter M. L. Murine MHC polymorphism and T cell specificities. Science. 1989 May 5;244(4904):572–575. doi: 10.1126/science.2470147. [DOI] [PubMed] [Google Scholar]
- Su H., Morrison R. P., Watkins N. G., Caldwell H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990 Jul 1;172(1):203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomai M., Kotb M., Majumdar G., Beachey E. H. Superantigenicity of streptococcal M protein. J Exp Med. 1990 Jul 1;172(1):359–362. doi: 10.1084/jem.172.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. M., Rogers M. V., Liew F. Y. Identification and characterization of host-protective T-cell epitopes of a major surface glycoprotein (gp63) from Leishmania major. Immunology. 1991 Jan;72(1):3–9. [PMC free article] [PubMed] [Google Scholar]


